Barcoding of GPCR trafficking and signaling through the various trafficking roadmaps by compartmentalized signaling networks
- PMID: 28449947
- PMCID: PMC5512170
- DOI: 10.1016/j.cellsig.2017.04.015
Barcoding of GPCR trafficking and signaling through the various trafficking roadmaps by compartmentalized signaling networks
Abstract
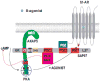
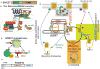
Proper signaling by G protein coupled receptors (GPCR) is dependent on the specific repertoire of transducing, enzymatic and regulatory kinases and phosphatases that shape its signaling output. Activation and signaling of the GPCR through its cognate G protein is impacted by G protein-coupled receptor kinase (GRK)-imprinted "barcodes" that recruit β-arrestins to regulate subsequent desensitization, biased signaling and endocytosis of the GPCR. The outcome of agonist-internalized GPCR in endosomes is also regulated by sequence motifs or "barcodes" within the GPCR that mediate its recycling to the plasma membrane or retention and eventual degradation as well as its subsequent signaling in endosomes. Given the vast number of diverse sequences in GPCR, several trafficking mechanisms for endosomal GPCR have been described. The majority of recycling GPCR, are sorted out of endosomes in a "sequence-dependent pathway" anchored around a type-1 PDZ-binding module found in their C-tails. For a subset of these GPCR, a second "barcode" imprinted onto specific GPCR serine/threonine residues by compartmentalized kinase networks was required for their efficient recycling through the "sequence-dependent pathway". Mutating the serine/threonine residues involved, produced dramatic effects on GPCR trafficking, indicating that they played a major role in setting the trafficking itinerary of these GPCR. While endosomal SNX27, retromer/WASH complexes and actin were required for efficient sorting and budding of all these GPCR, additional proteins were required for GPCR sorting via the second "barcode". Here we will review recent developments in GPCR trafficking in general and the human β1-adrenergic receptor in particular across the various trafficking roadmaps. In addition, we will discuss the role of GPCR trafficking in regulating endosomal GPCR signaling, which promote biochemical and physiological effects that are distinct from those generated by the GPCR signal transduction pathway in membranes.
Keywords: Endosomes; GPCR; GRK; PKA; SNX27; Signaling; Trafficking.
Copyright © 2017. Published by Elsevier Inc.
Figures
References
-
- Ellisdon AM, Halls ML. Compartmentalization of GPCR signalling controls unique cellular responses. Biochem Soc Trans. 2016;44:562–567. - PubMed
-
- Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–568. - PubMed
-
- Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

