Cholesterol Efflux Capacity, High-Density Lipoprotein Particle Number, and Incident Cardiovascular Events: An Analysis From the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin)
- PMID: 28450350
- PMCID: PMC5490983
- DOI: 10.1161/CIRCULATIONAHA.116.025678
Cholesterol Efflux Capacity, High-Density Lipoprotein Particle Number, and Incident Cardiovascular Events: An Analysis From the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin)
Abstract
Background: Recent failures of drugs that raised high-density lipoprotein (HDL) cholesterol levels to reduce cardiovascular events in clinical trials have led to increased interest in alternative indices of HDL quality, such as cholesterol efflux capacity, and HDL quantity, such as HDL particle number. However, no studies have directly compared these metrics in a contemporary population that includes potent statin therapy and low low-density lipoprotein cholesterol.
Methods: HDL cholesterol levels, apolipoprotein A-I, cholesterol efflux capacity, and HDL particle number were assessed at baseline and 12 months in a nested case-control study of the JUPITER trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin), a randomized primary prevention trial that compared rosuvastatin treatment to placebo in individuals with normal low-density lipoprotein cholesterol but increased C-reactive protein levels. In total, 314 cases of incident cardiovascular disease (CVD) (myocardial infarction, unstable angina, arterial revascularization, stroke, or cardiovascular death) were compared to age- and gender-matched controls. Conditional logistic regression models adjusting for risk factors evaluated associations between HDL-related biomarkers and incident CVD.
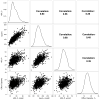
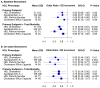
Results: Cholesterol efflux capacity was moderately correlated with HDL cholesterol, apolipoprotein A-I, and HDL particle number (Spearman r= 0.39, 0.48, and 0.39 respectively; P<0.001). Baseline HDL particle number was inversely associated with incident CVD (adjusted odds ratio per SD increment [OR/SD], 0.69; 95% confidence interval [CI], 0.56-0.86; P<0.001), whereas no significant association was found for baseline cholesterol efflux capacity (OR/SD, 0.89; 95% CI, 0.72-1.10; P=0.28), HDL cholesterol (OR/SD, 0.82; 95% CI, 0.66-1.02; P=0.08), or apolipoprotein A-I (OR/SD, 0.83; 95% CI, 0.67-1.03; P=0.08). Twelve months of rosuvastatin (20 mg/day) did not change cholesterol efflux capacity (average percentage change -1.5%, 95% CI, -13.3 to +10.2; P=0.80), but increased HDL cholesterol (+7.7%), apolipoprotein A-I (+4.3%), and HDL particle number (+5.2%). On-statin cholesterol efflux capacity was inversely associated with incident CVD (OR/SD, 0.62; 95% CI, 0.42-0.92; P=0.02), although HDL particle number again emerged as the strongest predictor (OR/SD, 0.51; 95% CI, 0.33-0.77; P<0.001).
Conclusions: In JUPITER, cholesterol efflux capacity was associated with incident CVD in individuals on potent statin therapy but not at baseline. For both baseline and on-statin analyses, HDL particle number was the strongest of 4 HDL-related biomarkers as an inverse predictor of incident events and biomarker of residual risk.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT00239681.
Keywords: cardiovascular disease risk factors; high-density lipoprotein cholesterol.
© 2017 American Heart Association, Inc.
Figures
Similar articles
-
Atherogenic Lipoprotein Subfractions Determined by Ion Mobility and First Cardiovascular Events After Random Allocation to High-Intensity Statin or Placebo: The Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) Trial.Circulation. 2015 Dec 8;132(23):2220-9. doi: 10.1161/CIRCULATIONAHA.115.016857. Epub 2015 Sep 25. Circulation. 2015. PMID: 26408274 Free PMC article. Clinical Trial.
-
High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy.Circulation. 2013 Sep 10;128(11):1189-97. doi: 10.1161/CIRCULATIONAHA.113.002671. Epub 2013 Sep 3. Circulation. 2013. PMID: 24002795 Free PMC article. Clinical Trial.
-
On-treatment non-high-density lipoprotein cholesterol, apolipoprotein B, triglycerides, and lipid ratios in relation to residual vascular risk after treatment with potent statin therapy: JUPITER (justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin).J Am Coll Cardiol. 2012 Apr 24;59(17):1521-8. doi: 10.1016/j.jacc.2011.12.035. J Am Coll Cardiol. 2012. PMID: 22516441 Free PMC article.
-
Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER)--can C-reactive protein be used to target statin therapy in primary prevention?Am J Cardiol. 2006 Jan 16;97(2A):33A-41A. doi: 10.1016/j.amjcard.2005.11.014. Epub 2005 Dec 1. Am J Cardiol. 2006. PMID: 16442935 Review.
-
Rosuvastatin, inflammation, C-reactive protein, JUPITER, and primary prevention of cardiovascular disease--a perspective.Drug Des Devel Ther. 2010 Dec 9;4:383-413. doi: 10.2147/DDDT.S10812. Drug Des Devel Ther. 2010. PMID: 21267417 Free PMC article. Review.
Cited by
-
Air Pollution: Another Threat to HDL Function.Int J Mol Sci. 2022 Dec 24;24(1):317. doi: 10.3390/ijms24010317. Int J Mol Sci. 2022. PMID: 36613760 Free PMC article. Review.
-
Statin dose reduction with complementary diet therapy: A pilot study of personalized medicine.Mol Metab. 2018 May;11:137-144. doi: 10.1016/j.molmet.2018.02.005. Epub 2018 Feb 20. Mol Metab. 2018. PMID: 29503145 Free PMC article. Clinical Trial.
-
Recent Updates and Advances in the Use of Glycated Albumin for the Diagnosis and Monitoring of Diabetes and Renal, Cerebro- and Cardio-Metabolic Diseases.J Clin Med. 2020 Nov 11;9(11):3634. doi: 10.3390/jcm9113634. J Clin Med. 2020. PMID: 33187372 Free PMC article. Review.
-
Dysfunctional HDL Diagnostic Metrics for Cardiovascular Disease Risk Stratification: Are we Ready to Implement in Clinics?J Cardiovasc Transl Res. 2025 Feb;18(1):169-184. doi: 10.1007/s12265-024-10559-x. Epub 2024 Sep 19. J Cardiovasc Transl Res. 2025. PMID: 39298091 Review.
-
Neutrophil subsets and their gene signature associate with vascular inflammation and coronary atherosclerosis in lupus.JCI Insight. 2018 Apr 19;3(8):e99276. doi: 10.1172/jci.insight.99276. eCollection 2018 Apr 19. JCI Insight. 2018. PMID: 29669944 Free PMC article. Clinical Trial.
References
-
- Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–73. - PubMed
-
- AIM-HIGH Investigators. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. - PubMed
-
- HPS2-THRIVE Collaborative Group. Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–212. - PubMed
-
- Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS dal-OUTCOMES Investigators. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

