MerTK expressing hepatic macrophages promote the resolution of inflammation in acute liver failure
- PMID: 28450389
- PMCID: PMC5868289
- DOI: 10.1136/gutjnl-2016-313615
MerTK expressing hepatic macrophages promote the resolution of inflammation in acute liver failure
Abstract
Objective: Acute liver failure (ALF) is characterised by overwhelming hepatocyte death and liver inflammation with massive infiltration of myeloid cells in necrotic areas. The mechanisms underlying resolution of acute hepatic inflammation are largely unknown. Here, we aimed to investigate the impact of Mer tyrosine kinase (MerTK) during ALF and also examine how the microenvironmental mediator, secretory leucocyte protease inhibitor (SLPI), governs this response.
Design: Flow cytometry, immunohistochemistry, confocal imaging and gene expression analyses determined the phenotype, functional/transcriptomic profile and tissue topography of MerTK+ monocytes/macrophages in ALF, healthy and disease controls. The temporal evolution of macrophage MerTK expression and its impact on resolution was examined in APAP-induced acute liver injury using wild-type (WT) and Mer-deficient (Mer-/-) mice. SLPI effects on hepatic myeloid cells were determined in vitro and in vivo using APAP-treated WT mice.
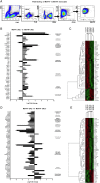
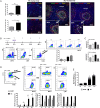
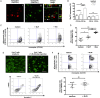
Results: We demonstrate a significant expansion of resolution-like MerTK+HLA-DRhigh cells in circulatory and tissue compartments of patients with ALF. Compared with WT mice which show an increase of MerTK+MHCIIhigh macrophages during the resolution phase in ALF, APAP-treated Mer-/- mice exhibit persistent liver injury and inflammation, characterised by a decreased proportion of resident Kupffer cells and increased number of neutrophils. Both in vitro and in APAP-treated mice, SLPI reprogrammes myeloid cells towards resolution responses through induction of a MerTK+HLA-DRhigh phenotype which promotes neutrophil apoptosis and their subsequent clearance.
Conclusions: We identify a hepatoprotective, MerTK+, macrophage phenotype that evolves during the resolution phase following ALF and represents a novel immunotherapeutic target to promote resolution responses following acute liver injury.
Keywords: ACUTE LIVER FAILURE; IMMUNOLOGY; INFLAMMATION; MACROPHAGES.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Competing interests: None declared.
Figures





Comment in
-
Repair macrophages in acute liver failure.Gut. 2018 Feb;67(2):202-203. doi: 10.1136/gutjnl-2017-314245. Epub 2017 May 8. Gut. 2018. PMID: 28483781 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
