Phosphoproteome Analysis Reveals Differential Mode of Action of Sorafenib in Wildtype and Mutated FLT3 Acute Myeloid Leukemia (AML) Cells
- PMID: 28450419
- PMCID: PMC5500767
- DOI: 10.1074/mcp.M117.067462
Phosphoproteome Analysis Reveals Differential Mode of Action of Sorafenib in Wildtype and Mutated FLT3 Acute Myeloid Leukemia (AML) Cells
Abstract
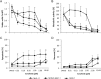
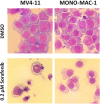
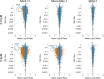
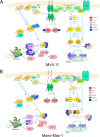
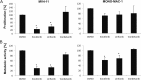
Constitutively activating internal tandem duplication (ITD) alterations of the receptor tyrosine kinase FLT3 (Fms-like tyrosine kinase 3) are common in acute myeloid leukemia (AML) and classifies FLT3 as an attractive therapeutic target. So far, applications of FLT3 small molecule inhibitors have been investigated primarily in FLT3-ITD+ patients. Only recently, a prolonged event-free survival has been observed in AML patients who were treated with the multikinase inhibitor sorafenib in addition to standard therapy. Here, we studied the sorafenib effect on proliferation in a panel of 13 FLT3-ITD- and FLT3-ITD+ AML cell lines. Sorafenib IC50 values ranged from 0.001 to 5.6 μm, whereas FLT3-ITD+ cells (MOLM-13, MV4-11) were found to be more sensitive to sorafenib than FLT3-ITD- cells. However, we identified two FLT3-ITD- cell lines (MONO-MAC-1 and OCI-AML-2) which were also sorafenib sensitive. Phosphoproteome analyses revealed that the affected pathways differed in sorafenib sensitive FLT3-ITD- and FLT3-ITD+ cells. In MV4-11 cells sorafenib suppressed mTOR signaling by direct inhibition of FLT3. In MONO-MAC-1 cells sorafenib inhibited the MEK/ERK pathway. These data suggest that the FLT3 status in AML patients might not be the only factor predicting response to treatment with sorafenib.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
Sorafenib induces paradoxical phosphorylation of the extracellular signal-regulated kinase pathway in acute myeloid leukemia cells lacking FLT3-ITD mutation.Leuk Lymphoma. 2015;56(9):2690-8. doi: 10.3109/10428194.2014.1003055. Epub 2015 Mar 3. Leuk Lymphoma. 2015. PMID: 25665465 Free PMC article.
-
Reversal of acquired drug resistance in FLT3-mutated acute myeloid leukemia cells via distinct drug combination strategies.Clin Cancer Res. 2014 May 1;20(9):2363-74. doi: 10.1158/1078-0432.CCR-13-2052. Epub 2014 Mar 11. Clin Cancer Res. 2014. PMID: 24619500 Free PMC article.
-
NFATc1 as a therapeutic target in FLT3-ITD-positive AML.Leukemia. 2015 Jul;29(7):1470-7. doi: 10.1038/leu.2015.95. Epub 2015 Apr 14. Leukemia. 2015. PMID: 25976987
-
Quizartinib (AC220): a promising option for acute myeloid leukemia.Drug Des Devel Ther. 2019 Apr 8;13:1117-1125. doi: 10.2147/DDDT.S198950. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31114157 Free PMC article. Review.
-
FLT3 inhibitors in the treatment of acute myeloid leukemia: current status and future perspectives.Minerva Med. 2020 Oct;111(5):427-442. doi: 10.23736/S0026-4806.20.06989-X. Epub 2020 Sep 21. Minerva Med. 2020. PMID: 32955823 Review.
Cited by
-
Role of drug transporters in the sensitivity of acute myeloid leukemia to sorafenib.Oncotarget. 2018 Jun 19;9(47):28474-28485. doi: 10.18632/oncotarget.25494. eCollection 2018 Jun 19. Oncotarget. 2018. PMID: 29983874 Free PMC article.
-
An atlas of bloodstream-accessible bone marrow proteins for site-directed therapy of acute myeloid leukemia.Leukemia. 2018 Feb;32(2):510-519. doi: 10.1038/leu.2017.208. Epub 2017 Jun 30. Leukemia. 2018. PMID: 28663580
-
Advancements in Oncoproteomics Technologies: Treading toward Translation into Clinical Practice.Proteomes. 2023 Jan 10;11(1):2. doi: 10.3390/proteomes11010002. Proteomes. 2023. PMID: 36648960 Free PMC article. Review.
-
Phosphotyrosine-based Phosphoproteomics for Target Identification and Drug Response Prediction in AML Cell Lines.Mol Cell Proteomics. 2020 May;19(5):884-899. doi: 10.1074/mcp.RA119.001504. Epub 2020 Feb 26. Mol Cell Proteomics. 2020. PMID: 32102969 Free PMC article.
-
Application of omics in the diagnosis, prognosis, and treatment of acute myeloid leukemia.Biomark Res. 2024 Jun 10;12(1):60. doi: 10.1186/s40364-024-00600-1. Biomark Res. 2024. PMID: 38858750 Free PMC article. Review.
References
-
- Levis M., Ravandi F., Wang E. S., Baer M. R., Perl A., Coutre S., Erba H., Stuart R. K., Baccarani M., Cripe l. D., Tallman M. S., Meloni G., Godley L. A., Langston A. A., Amadori S., Lewis I. D., Nagler A., Stone R., Yee K., Advani A., Douer D., Wiktor-Jedrzejczak W., Juliusson G., Litzow M. R., Petersdorf S., Sanz M., Kantarjian H. M., Sato T., Tremmel L., Bensen-Kennedy D. M., Small D., and Smith B. D. (2011) Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse. Blood 117, 3294–3301 - PMC - PubMed
-
- Fischer T., Stone R. M., Deangelo D. J., Galinsky I., Estey E., Lanza C., Fox E., Ehninger G., Feldman E. J., Schiller G. J., Klimek V. M., Nimer S. D., Gilliland D. G., Dutreix C., Huntsman-Labed A., Virkus J., and Giles F. J. (2010) Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol. 28, 4339–4345 - PMC - PubMed
-
- Cortes J. E., Kantarjian H., Foran J. M., Ghirdaladze D., Zodelava M., Borthakur G., Gammon G., Trone D., Armstrong R. C., James J., and Levis M. (2013) Phase I study of quizartinib administered daily to patients with relapsed or refractory acute myeloid leukemia irrespective of FMS-like tyrosine kinase 3-internal tandem duplication status. J Clin Oncol. 31, 3681–3687 - PMC - PubMed
-
- Wilhelm S., Carter C., Lynch M., Lowinger T., Dumas J., Smith R. A., Schwartz B., Simantov R., and Kelley S. (2006) Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat. Rev. Drug Discov. 5, 835–844 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

