Comparative pharmacology of a new recombinant FSH expressed by a human cell line
- PMID: 28450423
- PMCID: PMC5510450
- DOI: 10.1530/EC-17-0067
Comparative pharmacology of a new recombinant FSH expressed by a human cell line
Abstract
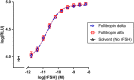
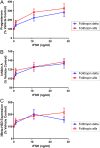
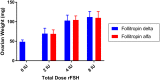
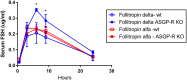
Recombinant FSH proteins are important therapeutic agents for the treatment of infertility, including follitropin alfa expressed in Chinese Hamster Ovary (CHO) cells and, more recently, follitropin delta expressed in the human cell line PER.C6. These recombinant FSH proteins have distinct glycosylation, and have distinct pharmacokinetic and pharmacodynamic profiles in women. Comparative experiments demonstrated that follitropin delta and follitropin alfa displayed the same in vitro potency at the human FSH receptor, but varied in their pharmacokinetics in mouse and rat. While follitropin delta clearance from serum depended in part on the hepatic asialoglycoprotein receptor (ASGPR), follitropin alfa clearance was unaffected by ASGPR inhibition in rat or genetic ablation in mice. The distinct properties of follitropin delta and follitropin alfa are likely to contribute to the differing pharmacokinetic and pharmacodynamic profiles observed in women and to influence their efficacy in therapeutic protocols for the treatment of infertility.
Keywords: FSH glycosylation; follicle-stimulating hormone; follitropin delta; gonadotropin clearance; recombinant FSH.
© 2017 The authors.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

