Circulating Cell-Free DNA to Guide Prostate Cancer Treatment with PARP Inhibition
- PMID: 28450425
- PMCID: PMC6143169
- DOI: 10.1158/2159-8290.CD-17-0261
Circulating Cell-Free DNA to Guide Prostate Cancer Treatment with PARP Inhibition
Abstract
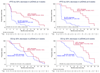
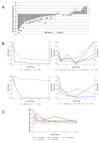
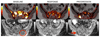
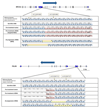
Biomarkers for more precise patient care are needed in metastatic prostate cancer. We have reported a phase II trial (TOPARP-A) of the PARP inhibitor olaparib in metastatic prostate cancer, demonstrating antitumor activity associating with homologous recombination DNA repair defects. We now report targeted and whole-exome sequencing of serial circulating cell-free DNA (cfDNA) samples collected during this trial. Decreases in cfDNA concentration independently associated with outcome in multivariable analyses (HR for overall survival at week 8: 0.19; 95% CI, 0.06-0.56; P = 0.003). All tumor tissue somatic DNA repair mutations were detectable in cfDNA; allele frequency of somatic mutations decreased selectively in responding patients (χ2P < 0.001). At disease progression, following response to olaparib, multiple subclonal aberrations reverting germline and somatic DNA repair mutations (BRCA2, PALB2) back in frame emerged as mechanisms of resistance. These data support the role of liquid biopsies as a predictive, prognostic, response, and resistance biomarker in metastatic prostate cancer.Significance: We report prospectively planned, serial, cfDNA analyses from patients with metastatic prostate cancer treated on an investigator-initiated phase II trial of olaparib. These analyses provide predictive, prognostic, response, and resistance data with "second hit" mutations first detectable at disease progression, suggesting clonal evolution from treatment-selective pressure and platinum resistance. Cancer Discov; 7(9); 1006-17. ©2017 AACR.See related commentary by Domchek, p. 937See related article by Kondrashova et al., p. 984See related article by Quigley et al., p. 999This article is highlighted in the In This Issue feature, p. 920.
©2017 American Association for Cancer Research.
Conflict of interest statement
JG, JM, WY, HM, NP, SM, RPL, DD, GF, BE, PF, GS, DNR, GB, CB, MA, MC, MC, IF, RR, SS, PR, ZZ, AS, NT, DV, AG, CJD, EH, SC and JSDB are employees of The Institute of Cancer Research, which is a joint applicant for the patent entitled ‘DNA damage repair inhibitors for treatment of cancer’ which includes the granted application US8143241. C. Lord reports holding patents related to the use of PARP inhibitors (WO2008020180 [A2] and WO2009027650 [A1]). J.S. de Bono has served as an advisor for AZ, Medivation, Pfizer, Merck, Tesaro and Biomarin. No other potential conflict of interest relevant to this article was reported.
Figures

Comment in
-
Prostate cancer: Circulating free DNA as biomarker.Nat Rev Urol. 2017 Jul;14(7):390. doi: 10.1038/nrurol.2017.74. Epub 2017 May 16. Nat Rev Urol. 2017. PMID: 28508881 No abstract available.
-
Reversion Mutations with Clinical Use of PARP Inhibitors: Many Genes, Many Versions.Cancer Discov. 2017 Sep;7(9):937-939. doi: 10.1158/2159-8290.CD-17-0734. Cancer Discov. 2017. PMID: 28864639
References
Publication types
MeSH terms
Substances
Grants and funding
- C1491/A15955/CRUK_/Cancer Research UK/United Kingdom
- MR/M003272/1/MRC_/Medical Research Council/United Kingdom
- MR/M018318/1/MRC_/Medical Research Council/United Kingdom
- 14276/CRUK_/Cancer Research UK/United Kingdom
- DH_/Department of Health/United Kingdom
- MR/M018618/1/MRC_/Medical Research Council/United Kingdom
- 13230/CRUK_/Cancer Research UK/United Kingdom
- C12540/A12829/CRUK_/Cancer Research UK/United Kingdom
- 20447/CRUK_/Cancer Research UK/United Kingdom
- C12540/A13230/CRUK_/Cancer Research UK/United Kingdom
- 12829/CRUK_/Cancer Research UK/United Kingdom
- C1491/A9895/CRUK_/Cancer Research UK/United Kingdom
- 24439/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

