Quality of reporting in abstracts of RCTs published in emergency medicine journals: a protocol for a systematic survey of the literature
- PMID: 28450467
- PMCID: PMC5566942
- DOI: 10.1136/bmjopen-2016-014981
Quality of reporting in abstracts of RCTs published in emergency medicine journals: a protocol for a systematic survey of the literature
Abstract
Introduction: The quality of reporting of abstracts of randomised controlled trials (RCTs) in major general medical journals and in some category-specific journals was shown to be poor before the publication of the ConsolidatedStandards of ReportingTrials (CONSORT) extension for abstracts in 2008, and an improvement in the quality of reporting of abstracts was observed after its publication. The effect of the publication of the CONSORT extension for abstracts on the quality of reporting of RCTs in emergency medicine journals has not been studied. In this paper, we present the protocol of a systematic survey of the literature, aimed at assessing the quality of reporting in abstracts of RCTs published in emergency medicine journals and at evaluating the effect of the publication of the CONSORT extension for abstracts on the quality of reporting.
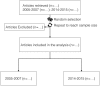
Methods and analysis: The Medline database will be searched for RCTs published in the years 2005-2007 and 2014-2015 in the top 10 emergency medicine journals, according to their impact factor. Candidate studies will be screened for inclusion in the review. Exclusion criteria will be the following: the abstract is not available, they are published only as abstracts, still recruiting, or duplicate publications. The study outcomes will be the overall quality of reporting (number of items reported) according to the CONSORT extension and the compliance with its individual items. Two independent reviewers will screen each article for inclusion and will extract data on the CONSORT items and on other variables, which can possibly affect the quality of reporting.
Ethics and dissemination: This is a library-based study and therefore exempt from research ethics board review. The review results will be disseminated through abstract submission to conferences and publication in a peer-reviewed biomedical journal.
Keywords: Abstract; Accident & emergency medicine; Quality of reporting; Research methodology; Statistics &research methods.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Reporting quality of abstracts of trials published in top five pain journals: a protocol for a systematic survey.BMJ Open. 2016 Nov 21;6(11):e012319. doi: 10.1136/bmjopen-2016-012319. BMJ Open. 2016. PMID: 27872116 Free PMC article.
-
Assessment of the quality of reporting of randomised controlled trials in otorhinolaryngologic literature - adherence to the CONSORT statement.PLoS One. 2015 Mar 20;10(3):e0122328. doi: 10.1371/journal.pone.0122328. eCollection 2015. PLoS One. 2015. PMID: 25793517 Free PMC article.
-
Reporting Quality of Randomized Controlled Trials of Periodontal Diseases in Journal Abstracts-A Cross-sectional Survey and Bibliometric Analysis.J Evid Based Dent Pract. 2018 Jun;18(2):130-141.e22. doi: 10.1016/j.jebdp.2017.08.005. Epub 2017 Sep 21. J Evid Based Dent Pract. 2018. PMID: 29747793
-
Quality of reporting for pilot randomized controlled trials in the pediatric urology literature-A systematic review.J Pediatr Urol. 2021 Dec;17(6):846-854. doi: 10.1016/j.jpurol.2021.09.012. Epub 2021 Sep 24. J Pediatr Urol. 2021. PMID: 34635440
-
Quality of reporting in abstracts of randomized controlled trials published in leading journals of periodontology and implant dentistry: a survey.J Periodontol. 2012 Oct;83(10):1251-6. doi: 10.1902/jop.2012.110609. Epub 2012 Feb 14. J Periodontol. 2012. PMID: 22220771 Review.
Cited by
-
The TargetMine Data Warehouse: Enhancement and Updates.Front Genet. 2019 Oct 9;10:934. doi: 10.3389/fgene.2019.00934. eCollection 2019. Front Genet. 2019. PMID: 31649722 Free PMC article.
-
Did an introduction of CONSORT for abstracts guidelines improve reporting quality of randomised controlled trials' abstracts on Helicobacter pylori infection? Observational study.BMJ Open. 2022 Mar 30;12(3):e054978. doi: 10.1136/bmjopen-2021-054978. BMJ Open. 2022. PMID: 35354625 Free PMC article.
-
A comprehensive quality analysis of randomized controlled clinical trials of Asian ginseng and American ginseng based on the CONSORT guideline.J Ginseng Res. 2022 Jan;46(1):71-78. doi: 10.1016/j.jgr.2021.05.003. Epub 2021 May 18. J Ginseng Res. 2022. PMID: 35035241 Free PMC article.
References
-
- Barry HC, Ebell MH, Shaughnessy AF, et al. . Family physicians’ use of medical abstracts to guide decision making: style or substance? J Am Board Fam Pract 2001;14:437–42. - PubMed
-
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration, 2011; http://handbook.cochrane.org
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases