Sodium Channel Nav1.8 Underlies TTX-Resistant Axonal Action Potential Conduction in Somatosensory C-Fibers of Distal Cutaneous Nerves
- PMID: 28450535
- PMCID: PMC5444201
- DOI: 10.1523/JNEUROSCI.3799-16.2017
Sodium Channel Nav1.8 Underlies TTX-Resistant Axonal Action Potential Conduction in Somatosensory C-Fibers of Distal Cutaneous Nerves
Abstract
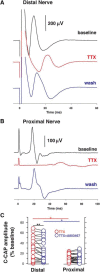
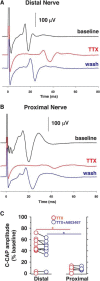
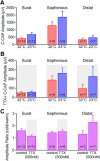
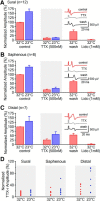
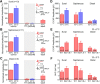
Voltage-gated sodium (NaV) channels are responsible for the initiation and conduction of action potentials within primary afferents. The nine NaV channel isoforms recognized in mammals are often functionally divided into tetrodotoxin (TTX)-sensitive (TTX-s) channels (NaV1.1-NaV1.4, NaV1.6-NaV1.7) that are blocked by nanomolar concentrations and TTX-resistant (TTX-r) channels (NaV1.8 and NaV1.9) inhibited by millimolar concentrations, with NaV1.5 having an intermediate toxin sensitivity. For small-diameter primary afferent neurons, it is unclear to what extent different NaV channel isoforms are distributed along the peripheral and central branches of their bifurcated axons. To determine the relative contribution of TTX-s and TTX-r channels to action potential conduction in different axonal compartments, we investigated the effects of TTX on C-fiber-mediated compound action potentials (C-CAPs) of proximal and distal peripheral nerve segments and dorsal roots from mice and pigtail monkeys (Macaca nemestrina). In the dorsal roots and proximal peripheral nerves of mice and nonhuman primates, TTX reduced the C-CAP amplitude to 16% of the baseline. In contrast, >30% of the C-CAP was resistant to TTX in distal peripheral branches of monkeys and WT and NaV1.9-/- mice. In nerves from NaV1.8-/- mice, TTX-r C-CAPs could not be detected. These data indicate that NaV1.8 is the primary isoform underlying TTX-r conduction in distal axons of somatosensory C-fibers. Furthermore, there is a differential spatial distribution of NaV1.8 within C-fiber axons, being functionally more prominent in the most distal axons and terminal regions. The enrichment of NaV1.8 in distal axons may provide a useful target in the treatment of pain of peripheral origin.SIGNIFICANCE STATEMENT It is unclear whether individual sodium channel isoforms exert differential roles in action potential conduction along the axonal membrane of nociceptive, unmyelinated peripheral nerve fibers, but clarifying the role of sodium channel subtypes in different axonal segments may be useful for the development of novel analgesic strategies. Here, we provide evidence from mice and nonhuman primates that a substantial portion of the C-fiber compound action potential in distal peripheral nerves, but not proximal nerves or dorsal roots, is resistant to tetrodotoxin and that, in mice, this effect is mediated solely by voltage-gated sodium channel 1.8 (NaV1.8). The functional prominence of NaV1.8 within the axonal compartment immediately proximal to its termination may affect strategies targeting pain of peripheral origin.
Keywords: nociceptor; nonhuman primate; pain; sodium channels.
Copyright © 2017 the authors 0270-6474/17/375205-11$15.00/0.
Figures
Similar articles
-
Role of TTX-sensitive and TTX-resistant sodium channels in Adelta- and C-fiber conduction and synaptic transmission.J Neurophysiol. 2008 Feb;99(2):617-28. doi: 10.1152/jn.00944.2007. Epub 2007 Dec 5. J Neurophysiol. 2008. PMID: 18057109
-
Different role of TTX-sensitive voltage-gated sodium channel (NaV 1) subtypes in action potential initiation and conduction in vagal airway nociceptors.J Physiol. 2018 Apr 15;596(8):1419-1432. doi: 10.1113/JP275698. J Physiol. 2018. PMID: 29435993 Free PMC article.
-
Contactin regulates the current density and axonal expression of tetrodotoxin-resistant but not tetrodotoxin-sensitive sodium channels in DRG neurons.Eur J Neurosci. 2005 Jul;22(1):39-49. doi: 10.1111/j.1460-9568.2005.04186.x. Eur J Neurosci. 2005. PMID: 16029194
-
Blocking voltage-gated sodium channels as a strategy to suppress pathological cough.Pulm Pharmacol Ther. 2017 Dec;47:38-41. doi: 10.1016/j.pupt.2017.05.010. Epub 2017 May 15. Pulm Pharmacol Ther. 2017. PMID: 28522215 Review.
-
Voltage-gated sodium channels in the mammalian heart.Glob Cardiol Sci Pract. 2014 Dec 31;2014(4):449-63. doi: 10.5339/gcsp.2014.58. eCollection 2014. Glob Cardiol Sci Pract. 2014. PMID: 25780798 Free PMC article. Review.
Cited by
-
Modulation of human dorsal root ganglion neuron firing by the Nav1.8 inhibitor suzetrigine.Proc Natl Acad Sci U S A. 2025 Jun 3;122(22):e2503570122. doi: 10.1073/pnas.2503570122. Epub 2025 May 27. Proc Natl Acad Sci U S A. 2025. PMID: 40424150
-
The physiological function of different voltage-gated sodium channels in pain.Nat Rev Neurosci. 2021 May;22(5):263-274. doi: 10.1038/s41583-021-00444-w. Epub 2021 Mar 29. Nat Rev Neurosci. 2021. PMID: 33782571 Review.
-
The role of free fatty acid receptor pathways in a selective regulation of TRPA1 and TRPV1 by resolvins in primary sensory neurons.J Cell Physiol. 2022 Sep;237(9):3651-3660. doi: 10.1002/jcp.30826. Epub 2022 Jul 8. J Cell Physiol. 2022. PMID: 35802479 Free PMC article.
-
Controlling the "Opioid Epidemic": A Novel Chemical Entity (NCE) to Reduce or Supplant Opiate Use for Chronic Pain.J Psychiatr Brain Sci. 2020;5:e200022. doi: 10.20900/jpbs.20200022. Epub 2020 Oct 5. J Psychiatr Brain Sci. 2020. PMID: 33117893 Free PMC article.
-
A Novel Single-Domain Na+-Selective Voltage-Gated Channel in Photosynthetic Eukaryotes.Plant Physiol. 2020 Dec;184(4):1674-1683. doi: 10.1104/pp.20.00889. Epub 2020 Oct 1. Plant Physiol. 2020. PMID: 33004614 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
