Decreased Motor Neuron Support by SMA Astrocytes due to Diminished MCP1 Secretion
- PMID: 28450545
- PMCID: PMC5456111
- DOI: 10.1523/JNEUROSCI.3472-16.2017
Decreased Motor Neuron Support by SMA Astrocytes due to Diminished MCP1 Secretion
Abstract
Spinal muscular atrophy (SMA) is an autosomal-recessive disorder characterized by severe, often fatal muscle weakness due to loss of motor neurons. SMA patients have deletions and other mutations of the survival of motor neuron 1 (SMN1) gene, resulting in decreased SMN protein. Astrocytes are the primary support cells of the CNS and are responsible for glutamate clearance, metabolic support, response to injury, and regulation of signal transmission. Astrocytes have been implicated in SMA as in in other neurodegenerative disorders. Astrocyte-specific rescue of SMN protein levels has been shown to mitigate disease manifestations in mice. However, the mechanism by which SMN deficiency in astrocytes may contribute to SMA is unclear and what aspect of astrocyte activity is lacking is unknown. Therefore, it is worthwhile to identify defects in SMN-deficient astrocytes that compromise normal function. We show here that SMA astrocyte cultures derived from mouse spinal cord of both sexes are deficient in supporting both WT and SMN-deficient motor neurons derived from male, female, and mixed-sex sources and that this deficiency may be mitigated with secreted factors. In particular, SMN-deficient astrocytes have decreased levels of monocyte chemoactive protein 1 (MCP1) secretion compared with controls and MCP1 restoration stimulates outgrowth of neurites from cultured motor neurons. Correction of MCP1 deficiency may thus be a new therapeutic approach to SMA.SIGNIFICANCE STATEMENT Spinal muscular atrophy (SMA) is caused by the loss of motor neurons, but astrocyte dysfunction also contributes to the disease in mouse models. Monocyte chemoactive protein 1 (MCP1) has been shown to be neuroprotective and is released by astrocytes. Here, we report that MCP1 levels are decreased in SMA mice and that replacement of deficient MCP1 increases differentiation and neurite length of WT and SMN-deficient motor-neuron-like cells in cell culture. This study reveals a novel aspect of astrocyte dysfunction in SMA and indicates a possible approach for improving motor neuron growth and survival in this disease.
Keywords: MCP1/CCL2; astrocytes; iPSC; motor neuron; spinal muscular atrophy.
Copyright © 2017 the authors 0270-6474/17/375309-10$15.00/0.
Figures
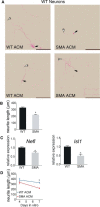
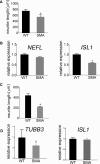

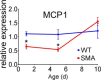

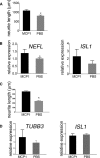


Comment in
-
Effects of Astroglia on Motor Neurons in Spinal Muscular Atrophy.J Neurosci. 2017 Sep 6;37(36):8578-8580. doi: 10.1523/JNEUROSCI.1578-17.2017. J Neurosci. 2017. PMID: 28878096 Free PMC article. No abstract available.
References
-
- Andjelkovic AV, Kerkovich D, Shanley J, Pulliam L, Pachter JS (1999) Expression of binding sites for beta chemokines on human astrocytes. Glia 28:225–235. - PubMed
-
- Aït-Ikhlef A, Hantaz-Ambroise D, Henderson CE, Rieger F (2000) Influence of factors secrAieted by wobbler astrocytes on neuronal and motoneuronal survival. J Neurosci Res 59:100–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
