Spiral Ganglion Neuron Projection Development to the Hindbrain in Mice Lacking Peripheral and/or Central Target Differentiation
- PMID: 28450830
- PMCID: PMC5389974
- DOI: 10.3389/fncir.2017.00025
Spiral Ganglion Neuron Projection Development to the Hindbrain in Mice Lacking Peripheral and/or Central Target Differentiation
Abstract
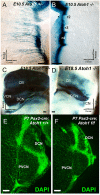
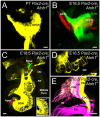
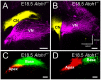
We investigate the importance of the degree of peripheral or central target differentiation for mouse auditory afferent navigation to the organ of Corti and auditory nuclei in three different mouse models: first, a mouse in which the differentiation of hair cells, but not central auditory nuclei neurons is compromised (Atoh1-cre; Atoh1f/f ); second, a mouse in which hair cell defects are combined with a delayed defect in central auditory nuclei neurons (Pax2-cre; Atoh1f/f ), and third, a mouse in which both hair cells and central auditory nuclei are absent (Atoh1-/-). Our results show that neither differentiated peripheral nor the central target cells of inner ear afferents are needed (hair cells, cochlear nucleus neurons) for segregation of vestibular and cochlear afferents within the hindbrain and some degree of base to apex segregation of cochlear afferents. These data suggest that inner ear spiral ganglion neuron processes may predominantly rely on temporally and spatially distinct molecular cues in the region of the targets rather than interaction with differentiated target cells for a crude topological organization. These developmental data imply that auditory neuron navigation properties may have evolved before auditory nuclei.
Keywords: Atoh1 mutation; auditory nuclei; development; ear; sensory epithelia; sensory neurons.
Figures

Similar articles
-
Conditional deletion of Atoh1 using Pax2-Cre results in viable mice without differentiated cochlear hair cells that have lost most of the organ of Corti.Hear Res. 2011 May;275(1-2):66-80. doi: 10.1016/j.heares.2010.12.002. Epub 2010 Dec 10. Hear Res. 2011. PMID: 21146598 Free PMC article.
-
Atoh1-lineal neurons are required for hearing and for the survival of neurons in the spiral ganglion and brainstem accessory auditory nuclei.J Neurosci. 2009 Sep 9;29(36):11123-33. doi: 10.1523/JNEUROSCI.2232-09.2009. J Neurosci. 2009. PMID: 19741118 Free PMC article.
-
Development in the Mammalian Auditory System Depends on Transcription Factors.Int J Mol Sci. 2021 Apr 18;22(8):4189. doi: 10.3390/ijms22084189. Int J Mol Sci. 2021. PMID: 33919542 Free PMC article. Review.
-
Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit.Dev Dyn. 2005 Nov;234(3):633-50. doi: 10.1002/dvdy.20551. Dev Dyn. 2005. PMID: 16145671 Free PMC article.
-
Auditory system development: primary auditory neurons and their targets.Annu Rev Neurosci. 2002;25:51-101. doi: 10.1146/annurev.neuro.25.112701.142849. Epub 2002 Feb 5. Annu Rev Neurosci. 2002. PMID: 12052904 Review.
Cited by
-
Wilhelm His' lasting insights into hindbrain and cranial ganglia development and evolution.Dev Biol. 2018 Dec 1;444 Suppl 1(Suppl 1):S14-S24. doi: 10.1016/j.ydbio.2018.02.001. Epub 2018 Feb 12. Dev Biol. 2018. PMID: 29447907 Free PMC article. Review.
-
Fzd3 Expression Within Inner Ear Afferent Neurons Is Necessary for Central Pathfinding.Front Neurosci. 2022 Jan 27;15:779871. doi: 10.3389/fnins.2021.779871. eCollection 2021. Front Neurosci. 2022. PMID: 35153658 Free PMC article.
-
Ptf1a expression is necessary for correct targeting of spiral ganglion neurons within the cochlear nuclei.Neurosci Lett. 2023 May 29;806:137244. doi: 10.1016/j.neulet.2023.137244. Epub 2023 Apr 11. Neurosci Lett. 2023. PMID: 37055006 Free PMC article.
-
Transplantation of Ears Provides Insights into Inner Ear Afferent Pathfinding Properties.Dev Neurobiol. 2018 Nov;78(11):1064-1080. doi: 10.1002/dneu.22629. Epub 2018 Sep 15. Dev Neurobiol. 2018. PMID: 30027559 Free PMC article.
-
Ear transplantations reveal conservation of inner ear afferent pathfinding cues.Sci Rep. 2018 Sep 14;8(1):13819. doi: 10.1038/s41598-018-31952-y. Sci Rep. 2018. PMID: 30218045 Free PMC article.
References
-
- Altman J., Bayer S. A. (1980). Development of the brain stem in the rat. III. Thymidine-radiographic study of the time of origin of neurons of the vestibular and auditory nuclei of the upper medulla. J. Comp. Neurol. 194, 877–904. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

