CaMKII: The molecular villain that aggravates cardiovascular disease
- PMID: 28450904
- PMCID: PMC5403363
- DOI: 10.3892/etm.2017.4034
CaMKII: The molecular villain that aggravates cardiovascular disease
Abstract
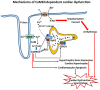
Pathological remodeling of the myocardium is an integral part of the events that lead to heart failure (HF), which involves altered gene expression, disturbed signaling pathways and altered Ca2+ homeostasis and the players involved in this process. Of particular interest is the chronic activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) isoforms in heart, which further aggravate the injury to myocardium. Expression and activity of CaMKII have been found to be elevated in various conditions of stressed myocardium and in different heart diseases in both animal models as well as heart patients. CaMKII is a signaling molecule that regulates many cellular pathways by phosphorylating several proteins involved in excitation-contraction coupling and relaxation events in heart, cardiomyocyte apoptosis, transcriptional activation of genes related to cardiac hypertrophy, inflammation, and arrhythmias. CaMKII is activated by reactive oxygen species (ROS), which are elevated under conditions of ischemia-reperfusion injury and in a cyclical manner, CaMKII in turn elevates ROS production. Both ROS and activated CaMKII increase Ca-induced Ca release from sarcoplasmic reticulum, which leads to cardiomyocyte membrane depolarization and arrhythmias. These CaMKII-mediated changes in heart ultimately culminate in dysfunctional myocardium and HF. Genetic studies in animal models clearly demonstrated that inactivation of CaMKII is protective against a variety of stress induced cardiac dysfunctions. Despite significant leaps in understanding the structural details of CaMKII, which is a very complicated and multimeric modular protein, currently there is no specific and potent inhibitor of this enzyme, that can be developed for therapeutic purposes.
Keywords: CaMKII; RyR2; SERCA; arrhythmias; cardiac dysfunction; cardiac hypertrophy; heart failure.
Figures

Similar articles
-
Ca2+/calmodulin-dependent protein kinase II and protein kinase A differentially regulate sarcoplasmic reticulum Ca2+ leak in human cardiac pathology.Circulation. 2013 Aug 27;128(9):970-81. doi: 10.1161/CIRCULATIONAHA.113.001746. Epub 2013 Jul 19. Circulation. 2013. PMID: 23877259
-
Calcium-calmodulin-dependent protein kinase mediates the intracellular signalling pathways of cardiac apoptosis in mice with impaired glucose tolerance.J Physiol. 2017 Jun 15;595(12):4089-4108. doi: 10.1113/JP273714. Epub 2017 Mar 2. J Physiol. 2017. PMID: 28105734 Free PMC article.
-
Chasing cardiac physiology and pathology down the CaMKII cascade.Am J Physiol Heart Circ Physiol. 2015 May 15;308(10):H1177-91. doi: 10.1152/ajpheart.00007.2015. Epub 2015 Mar 6. Am J Physiol Heart Circ Physiol. 2015. PMID: 25747749 Free PMC article. Review.
-
Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure.Circ Res. 2005 Dec 9;97(12):1314-22. doi: 10.1161/01.RES.0000194329.41863.89. Epub 2005 Nov 3. Circ Res. 2005. PMID: 16269653
-
Cardiomyocyte calcium and calcium/calmodulin-dependent protein kinase II: friends or foes?Recent Prog Horm Res. 2004;59:141-68. doi: 10.1210/rp.59.1.141. Recent Prog Horm Res. 2004. PMID: 14749501 Review.
Cited by
-
High uric acid promotes mitophagy through the ROS/CaMKIIδ/Parkin pathway in cardiomyocytes in vitro and in vivo.Am J Transl Res. 2021 Aug 15;13(8):8754-8765. eCollection 2021. Am J Transl Res. 2021. PMID: 34539992 Free PMC article.
-
Molecular Mechanisms of L-Type Calcium Channel Dysregulation in Heart Failure.Int J Mol Sci. 2025 Jun 15;26(12):5738. doi: 10.3390/ijms26125738. Int J Mol Sci. 2025. PMID: 40565202 Free PMC article. Review.
-
Non-canonical WNT signalling in cardiovascular disease: mechanisms and therapeutic implications.Nat Rev Cardiol. 2022 Dec;19(12):783-797. doi: 10.1038/s41569-022-00718-5. Epub 2022 Jun 13. Nat Rev Cardiol. 2022. PMID: 35697779 Free PMC article. Review.
-
Combined fructose and sucrose consumption from an early age aggravates cardiac oxidative damage and causes a dilated cardiomyopathy in SHR rats.J Clin Biochem Nutr. 2023 Nov;73(3):205-213. doi: 10.3164/jcbn.23-2. Epub 2023 Aug 9. J Clin Biochem Nutr. 2023. PMID: 37970552 Free PMC article.
-
Gene Therapy for Catecholaminergic Polymorphic Ventricular Tachycardia by Inhibition of Ca2+/Calmodulin-Dependent Kinase II.Circulation. 2019 Jul 30;140(5):405-419. doi: 10.1161/CIRCULATIONAHA.118.038514. Epub 2019 Jun 3. Circulation. 2019. PMID: 31155924 Free PMC article.
References
-
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, et al. ESC Committee for Practice Guidelines: Esc guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the heart failure association (hfa) of the esc. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
