Bupivacaine effectively relieves inflammation-induced pain by suppressing activation of the NF-κB signalling pathway and inhibiting the activation of spinal microglia and astrocytes
- PMID: 28450945
- PMCID: PMC5403393
- DOI: 10.3892/etm.2017.4058
Bupivacaine effectively relieves inflammation-induced pain by suppressing activation of the NF-κB signalling pathway and inhibiting the activation of spinal microglia and astrocytes
Abstract
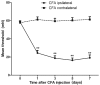
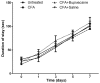
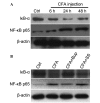
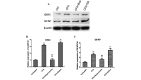
The pain induced by local acute inflammation results in mild to severe discomfort, in addition to the possibility of physiological dysfunction and psychiatric disorders, such as sleep disorders and depression. However, the pathogenesis of pain is yet to be fully elucidated. In the present study, the effects of bupivacaine were explored in rat models inflammatory pain in order to investigate the anti-pain mechanism of bupivacaine. Complete Freund's adjuvant (CFA) was injected into the right rear foot of the rats to establish a model of transient inflammation-induced pain. Rats were randomly divided into four groups (n=8): CFA, CFA plus bupivacaine, CFA plus saline and untreated. The mechanical withdrawal threshold (MWT) of the rats was detected prior to and following CFA injection, and the results demonstrated that the MWT in the right rear foot significantly decreased from the 1st day of CFA injection (P<0.01; n=8), as compared with the untreated controls. Bupivacaine treatment was demonstrated to significantly increase the MWT of rats treated with CFA stimulation, as compared with the CFA group (P<0.01). Rotarod testing was performed to assess the motor activity of the rats, and the results demonstrated no significant differences among the four groups (P>0.05). Furthermore, the respective body weights of the rats were determined every two days before and after CFA injection, and no significant differences were detected among the four groups (P>0.05). Western blot analysis was performed to analyze expression levels of IκB and nuclear factor (NF)-κB, and the results demonstrated that bupivacaine increased the expression of IκB and decreased the expression levels of NF-κB, as compared with the rats with CFA-induced inflammatory responses, suggesting that bupivacaine inhibited NF-κB activation in the dorsal horn of the lumbar spinal cord of the model rats. Furthermore, reverse transcription-quantitative polymerase chain reaction analysis was performed to analyze the expression levels of inflammatory cytokines, which demonstrated that bupivacaine significantly inhibited the expression of TNF-α, IL-1β and IL-6, as compared with the untreated group (P<0.01). Moreover, bupivacaine treatment significantly decreased the expression of spinal microglial marker OX42 and astrocyte marker-glial fibrillary acidic protein, as compared with the rats in the CFA group (P<0.01). The present findings demonstrated that treatment with bupivacaine significantly decreased the activation of microglia and astrocytes in rat models of inflammatory pain. Therefore, the present results may provide clarification of the pathogenesis and mechanism of inflammation-induced pain and may provide novel therapeutic strategies for the clinical treatment of pain.
Keywords: bupivacaine; glial fibrillary acidic protein; inflammatory cytokines; inflammatory pain; nuclear factor-κB; ox42.
Figures


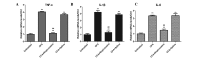
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
