Aberrant DNA methylation and expression of SPDEF and FOXA2 in airway epithelium of patients with COPD
- PMID: 28450970
- PMCID: PMC5404321
- DOI: 10.1186/s13148-017-0341-7
Aberrant DNA methylation and expression of SPDEF and FOXA2 in airway epithelium of patients with COPD
Abstract
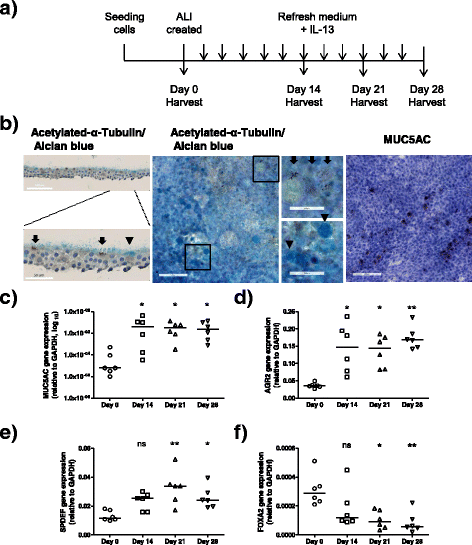
Background: Goblet cell metaplasia, a common feature of chronic obstructive pulmonary disease (COPD), is associated with mucus hypersecretion which contributes to the morbidity and mortality among patients. Transcription factors SAM-pointed domain-containing Ets-like factor (SPDEF) and forkhead box protein A2 (FOXA2) regulate goblet cell differentiation. This study aimed to (1) investigate DNA methylation and expression of SPDEF and FOXA2 during goblet cell differentiation and (2) compare this in airway epithelial cells from patients with COPD and controls during mucociliary differentiation.
Methods: To assess DNA methylation and expression of SPDEF and FOXA2 during goblet cell differentiation, primary airway epithelial cells, isolated from trachea (non-COPD controls) and bronchial tissue (patients with COPD), were differentiated by culture at the air-liquid interface (ALI) in the presence of cytokine interleukin (IL)-13 to promote goblet cell differentiation.
Results: We found that SPDEF expression was induced during goblet cell differentiation, while FOXA2 expression was decreased. Importantly, CpG number 8 in the SPDEF promoter was hypermethylated upon differentiation, whereas DNA methylation of FOXA2 promoter was not changed. In the absence of IL-13, COPD-derived ALI-cultured cells displayed higher SPDEF expression than control-derived ALI cultures, whereas no difference was found for FOXA2 expression. This was accompanied with hypomethylation of CpG number 6 in the SPDEF promoter and also hypomethylation of CpG numbers 10 and 11 in the FOXA2 promoter.
Conclusions: These findings suggest that aberrant DNA methylation of SPDEF and FOXA2 is one of the factors underlying mucus hypersecretion in COPD, opening new avenues for epigenetic-based inhibition of mucus hypersecretion.
Keywords: COPD; DNA methylation; FOXA2; Mucus; SPDEF.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

