Site-selective Suzuki-Miyaura coupling of heteroaryl halides - understanding the trends for pharmaceutically important classes
- PMID: 28451148
- PMCID: PMC5304707
- DOI: 10.1039/c6sc02118b
Site-selective Suzuki-Miyaura coupling of heteroaryl halides - understanding the trends for pharmaceutically important classes
Abstract
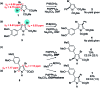
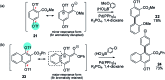
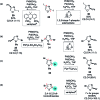
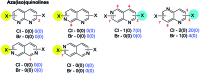
Suzuki-Miyaura cross-coupling reactions of heteroaryl polyhalides with aryl boronates are surveyed. Drawing on data from literature sources as well as bespoke searches of Pfizer's global chemistry RKB and CAS Scifinder® databases, the factors that determine the site-selectivity of these reactions are discussed with a view to rationalising the trends found.
Figures





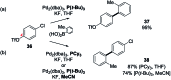



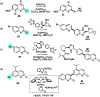

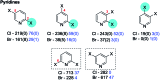
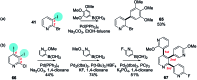
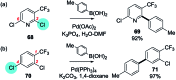





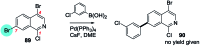
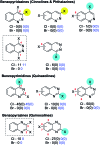
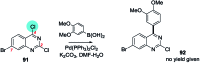
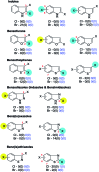
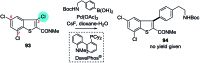

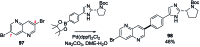
References
-
- Pozharskii A. F., Soldatenkov A. T. and Katritsky A. R., Heterocycles in Life and Society - An Introduction to Heterocyclic Chemistry and Biochemistry and the Role of Heterocycles in Science, Technology, Medicine and Agriculture, Wiley, Chichester, 1997.
-
- Kantak A. and DeBoef B., in Cross-Coupling and Heck-Type Reactions 3: Metal Catalyzed Heck-Type Reactions and C-C Cross Coupling via C-H Activation, ed. M. Larhed, Georg Thieme Verlag, Stuttgart-New York, 2012, vol. 3, pp. 585–641.
- Hirano K. and Miura M., in Sustainable Catalysis: Challenges and Practices for the Pharmaceutical and Fine Chemical Industries, ed. P. J. Dunn, K. K. M. Hii, M. J. Krische and M. T. Williams, Wiley-VCH, Weinheim, 2013, pp. 233–267.
- Petit A., Flygare J., Miller A. T., Winkel G., Ess D. H. Org. Lett. 2012;14:3680–3683. - PubMed
- Schranck J., Tlili A., Beller M. Angew. Chem., Int. Ed. 2014;53:9426–9428. - PubMed
- Taylor A. P., Robinson R. P., Fobian Y. M., Blakemore D. C., Jones L. H., Fadeyi O. Org. Biomol. Chem. 2016;14:6611–6637. - PubMed
-
-
In this context, a ‘pseudo-halogen’ is a functional group capable of undergoing oxidative addition (OA) with Pd(0) (e.g. a triflate). In this article the term ‘halide’ implicitly encompasses pseudo-halides
-
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

