Enantioselective bifunctional iminophosphorane catalyzed sulfa-Michael addition of alkyl thiols to unactivated β-substituted-α,β-unsaturated esters
- PMID: 28451207
- PMCID: PMC5358540
- DOI: 10.1039/c6sc02878k
Enantioselective bifunctional iminophosphorane catalyzed sulfa-Michael addition of alkyl thiols to unactivated β-substituted-α,β-unsaturated esters
Abstract
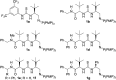
The highly enantioselective sulfa-Michael addition of alkyl thiols to unactivated β-substituted-α,β-unsaturated esters catalyzed by a bifunctional iminophosphorane (BIMP) organocatalyst is described. The low acidity of the alkyl thiol pro-nucleophiles is overcome by the high Brønsted basicity of the catalyst and the chiral scaffold/thiourea hydrogen-bond donor moiety provides the required enantiofacial discrimination in the addition step. The reaction is broad in scope with respect to the alkyl thiol and β-substituent of the α,β-unsaturated ester, affords sulfa-Michael adducts in excellent yields (up to >99%) and enantioselectivity (up to 97 : 3 er) and can operate down to 1 mol% catalyst loading.
Figures


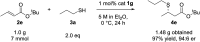

References
-
-
For reviews on organocatalysis, see:
- Dalko P. I., Moisan L. Angew. Chem., Int. Ed. 2004;43:5138. - PubMed
- Berkessel A. and Gröger H., Asymmetric Organocatalysis, Wiley VCH, Weinheim, 2005.
- List B., Yang J. W. Science. 2006;313:1584. - PubMed
- Dondoni A., Massi A. Angew. Chem., Int. Ed. 2008;47:4638. - PubMed
- Bertelsen S., Jørgensen K. A. Chem. Soc. Rev. 2009;38:2178. - PubMed
- Palomo C., Oiarbide M., López R. Chem. Soc. Rev. 2009;38:632. - PubMed
-
-
- Matsunaga S., Kinoshita T., Okada S., Harada S., Shibasaki M. J. Am. Chem. Soc. 2004;126:7559. - PubMed
- López F., Harutyunyan S. R., Meetsma A., Minnaard A. J., Feringa B. L. Angew. Chem., Int. Ed. 2005;44:2752. - PubMed
- Pubill-Ulldemolins C., Bonet A., Bo C., Gulyás H., Fernández E. Chem.–Eur. J. 2012;18:1121. - PubMed
-
- Tomioka K. and Nagaoka Y., Comprehensive Asymmetric Catalysis, ed. E. N. Jacobsen, A. Pfatlz and H. Yamamoto, Springer, New York, 1999, vol. 3.
-
-
For examples of enantioselective organocatalytic cycloaddition reactions using crotonate and cinnamate esters, see:
- Mathieu B., de Fays L., Ghosez L. Tetrahedron Lett. 2000;41:9561.
- Ryu D. H., Lee T. W., Corey E. J. J. Am. Chem. Soc. 2002;124:9992. - PubMed
- Gatzenmeier T., van Gemmeren M., Xie Y., Höfler D., Leutzsch M., List B. Science. 2016;351:949. - PubMed
-
-
- The electrophile-specific reactivity parameters E of trans-β-nitrostyrenes have been determined to be –15 < E < –12: Zenz I., Mayr H., J. Org. Chem., 2011, 76 , 9370 . - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

