Tailored oxido-vanadium(V) cage complexes for selective sulfoxidation in confined spaces
- PMID: 28451228
- PMCID: PMC5299934
- DOI: 10.1039/c6sc03045a
Tailored oxido-vanadium(V) cage complexes for selective sulfoxidation in confined spaces
Abstract
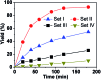
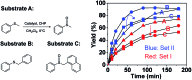
Five sets of oxido-vanadium(V) complexes, which include both cages and open structures, were prepared and tested in the catalytic oxidation of sulfides. It was found that the hemicryptophane complexes, which are simultaneously comprised of cyclotriveratrylene (CTV), binaphthol and oxido-vanadium(V) moieties, are the most efficient supramolecular catalysts. The specific shape of the confined hydrophobic space above the metal center leads to a strong improvement in the yield, selectivity and rate of the reaction, compared to the other catalysts investigated herein. A remarkable turnover number (TON) of 10 000 was obtained, which can be attributed to both the high reactivity and stability of the catalyst. Similarly to enzymes, the kinetic analysis shows that the mechanism of oxidation with the supramolecular catalysts obeys the Michaelis-Menten model, in which initial rate saturation occurs upon an increase in substrate concentration. This enzyme-like behavior is also supported by the competitive inhibition and substrate size-selectivity observed, which underline the crucial role played by the cavity.
Figures





References
-
- Raynal M., Ballester P., Vidal-Ferran A., Van Leeuwen P. W. N. M. Chem. Soc. Rev. 2014;43:1660. - PubMed
- Raynal M., Ballester P., Vidal-Ferran A., Van Leeuwen P. W. N. M. Chem. Soc. Rev. 2014;43:1734. - PubMed
- Brown C. J., Toste F. D., Bergman R. G., Raymond K. N. Chem. Rev. 2015;115:3012. - PubMed
- Pluth M. D., Bergman R. G., Raymond K. N. Acc. Chem. Res. 2009;42:1650. - PubMed
- Leenders S. H. A. M., Gramage-Doria R., de Bruin B., Reek J. N. H. Chem. Soc. Rev. 2015;44:433. - PubMed
- Breiner B., Clegg J. K., Nitschke J. R. Chem. Sci. 2011;2:51.
- Hyster T. K., Knörr L., Ward T. R., Rovis T. Science. 2012;338:500. - PMC - PubMed
- Heinisch T., Pellizzoni M., Dürrenberger M., Tinberg C. E., Köhler V., Klehr J., Schirmer T., Baker D., Ward T. R. J. Am. Chem. Soc. 2015;137:10414. - PubMed
- Hosseini M. W., Lehn J. M., Jones K. C., Plute K. E., Mertes K. B., Mertes M. P. J. Am. Chem. Soc. 1989;111:6330.
-
- Kohyama Y., Murase T., Fujita M. J. Am. Chem. Soc. 2014;136:2966. - PubMed
- Horiuchi S., Murase T., Fujita M. Angew. Chem., Int. Ed. 2012;51:12029. - PubMed
- Ikemoto K., Inokuma Y., Fujita M. J. Am. Chem. Soc. 2011;133:16806. - PubMed
- Nishioka Y., Yamaguchi T., Kawano M., Fujita M. J. Am. Chem. Soc. 2008;130:8160. - PubMed
- Wang Z. J., Clary K. N., Bergman R. G., Raymond K. N., Toste F. D. Nat. Chem. 2013;5:100. - PubMed
- Hart-Cooper W. M., Clary K. N., Toste F. D., Bergman R. G., Raymond K. N. J. Am. Chem. Soc. 2012;134:17873. - PubMed
- Hastings C. J., Fiedler D., Bergman R. G., Raymond K. N. J. Am. Chem. Soc. 2008;130:10977. - PubMed
- Wang Z. J., Brown C. J., Bergman R. G., Raymond K. N., Toste F. D. J. Am. Chem. Soc. 2011;133:7358. - PubMed
- Pluth M. D., Bergman R. G., Raymond K. N. Science. 2007;316:85. - PubMed
- Kaphan D. M., Levin M. D., Bergman R. G., Raymond K. N., Toste F. D. Science. 2015;350:1235. - PubMed
- Shenoy S. R., Crisostomo F. R. P., Iwasawa T., Rebek Jr J. J. Am. Chem. Soc. 2008;130:5658. - PubMed
- Smulders M. M. J., Nitschke J. R. Chem. Sci. 2012;3:785.
- Gramage-Doria R., Hessels J., Leenders S. H. A. M., Tröppner O., Dürr M., Ivanović-Burmazović I., Reek J. N. H. Angew. Chem., Int. Ed. 2014;53:13380. - PubMed
- Kersting B. Z. Anorg. Allg. Chem. 2004;630:765.
- Käss S., Gregor T., Kersting B. Angew. Chem., Int. Ed. 2006;45:101. - PubMed
- Steinfeld G., Lozan V., Kersting B. Angew. Chem., Int. Ed. 2003;42:2261. - PubMed
- Di Stefano S., Cacciapaglia R., Mandolini L. Eur. J. Org. Chem. 2014:7304. - PubMed
- Kataev E. A., Müller C. Tetrahedron. 2014;70:137.
-
- Wang Z. J., Brown C. J., Bergman R. G., Raymond K. N., Toste F. D. J. Am. Chem. Soc. 2011;133:7358. - PubMed
- Hastings C. J., Bergman R. G., Raymond K. N. Chem.–Eur. J. 2014;20:3966. - PubMed
- Hastings C. J., Pluth M. D., Bergman R. G., Raymond K. N. J. Am. Chem. Soc. 2010;132:6938. - PubMed
- Kopilevich S., Muller A., Weinstock I. A. J. Am. Chem. Soc. 2015;137:12740. - PubMed
-
- Hooley R. J. Nat. Chem. 2016;8:202. - PubMed
- Wang Q.-Q., Gonell S., Leenders S. H. A. M., Dürr M., Ivanović-Burmazović I., Reek J. N. H. Nat. Chem. 2016;8:225. - PubMed
- Cullen W., Misuraca M. C., Hunter C. A., Williams N. H., Ward M. D. Nat. Chem. 2016;8:231. - PubMed
- Sanders J. K. Chem.–Eur. J. 1998;4:1378.
- Yoshizawa M., Tamura M., Fujita M. Science. 2006;312:251. - PubMed
- Kang J., Rebek Jr J. Nature. 1997;385:50. - PubMed
- Mosca S., Yu Y., Gavette J. V., Zhang K. D., Rebek Jr J. J. Am. Chem. Soc. 2015;137:14582. - PubMed
-
- Brown C. J., Miller G. M., Johnson M. W., Bergman R. G., Raymond K. N. J. Am. Chem. Soc. 2011;133:11964. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

