Reversible ratiometric detection of highly reactive hydropersulfides using a FRET-based dual emission fluorescent probe
- PMID: 28451253
- PMCID: PMC5369533
- DOI: 10.1039/c6sc03856e
Reversible ratiometric detection of highly reactive hydropersulfides using a FRET-based dual emission fluorescent probe
Abstract
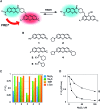
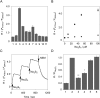
Hydropersulfide (R-SSH) is an important class of reactive sulfur species (RSS) involved in a variety of physiological processes in mammals. A fluorescent probe capable of real-time detection of hydropersulfide levels in living cells would be a versatile tool to elucidate its roles in cell signalling and redox homeostasis. In this paper, we report a ratiometric fluorescent probe for hydropersulfide sensing, based on a fluorescence resonance energy transfer (FRET) mechanism. This sensing mechanism involves a nucleophilic reaction of a hydropersulfide with the pyronine-unit of the probe, which modulates the intramolecular FRET efficiency to induce a dual-emission change. The reversible nature of this reaction allows us to detect increases and decreases of hydropersulfide levels in a real-time manner. The probe fluorometrically sensed highly reactive hydropersulfides, such as H2S2 and Cys-SSH, while the fluorescence response to biologically abundant cysteine and glutathione was negligible. Taking advantage of the reversible and selective sensing properties, this probe was successfully applied to the ratiometric imaging of concentration dynamics of endogenously produced hydropersulfides in living cells.
Figures

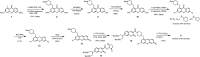


References
-
- Paul B. D., Snyder S. H. Nat. Rev. Mol. Cell Biol. 2012;13:499–507. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

