Activation and characterization of a cryptic gene cluster reveals a cyclization cascade for polycyclic tetramate macrolactams
- PMID: 28451290
- PMCID: PMC5361873
- DOI: 10.1039/c6sc03875a
Activation and characterization of a cryptic gene cluster reveals a cyclization cascade for polycyclic tetramate macrolactams
Abstract
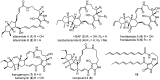
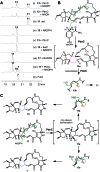
Polycyclic tetramate macrolactams (PTMs) are a growing class of natural products and are derived from a hybrid polyketide synthase (PKS)/non-ribosomal peptide synthetase (NRPS) pathway. PTM biosynthetic gene clusters are conserved and widely distributed in bacteria, however, most of them remain silent. Herein we report the activation of a PTM gene cluster in marine-derived Streptomyces pactum SCSIO 02999 by promoter engineering and heterologous expression, leading to the discovery of six new PTMs, pactamides A-F (11-16), with potent cytotoxic activity upon several human cancer cell lines. In vivo gene disruption experiments and in vitro biochemical assays reveal a reductive cyclization cascade for polycycle formation, with reactions sequentially generating the 5, 5/5 and 5/5/6 carbocyclic ring systems, catalysed by the phytoene dehydrogenase PtmB2, the oxidoreductase PtmB1, and the alcohol dehydrogenase PtmC, respectively. Furthermore, PtmC was demonstrated as a bifunctional cyclase for catalyzing the formation of the inner five-membered ring in ikarugamycin. This study suggests the possibility of finding more bioactive PTMs by genome mining and discloses a general mechanism for the formation of 5/5/6-type carbocyclic rings in PTMs.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

