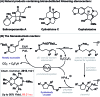
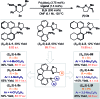
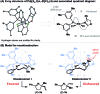
Enantioselective Narasaka-Heck cyclizations: synthesis of tetrasubstituted nitrogen-bearing stereocenters
- PMID: 28451314
- PMCID: PMC5390761
- DOI: 10.1039/c6sc04466b
Enantioselective Narasaka-Heck cyclizations: synthesis of tetrasubstituted nitrogen-bearing stereocenters
Abstract
The first examples of highly enantioselective Narasaka-Heck cyclizations are described. A SPINOL-derived P,N-ligand system enables Pd-catalyzed 5-exo cyclization of a range of oxime esters with sterically diverse trisubstituted alkenes to generate dihydropyrroles containing tetrasubstituted nitrogen-bearing stereocenters in 56 to 86% yield and 90 : 10 to 95 : 5 e.r. These processes are rare examples of reactions that proceed via enantioselective migratory insertion of alkenes into Pd-N bonds, and the first where trisubstituted alkenes are used to generate tetrasubstituted stereocenters with high enantioselectivity.
Figures
References
-
- Sato Y., Sodeoka M., Shibasaki M. J. Org. Chem. 1989;54:4738.
- Carpenter N. E., Kucera D. J., Overman L. E. J. Org. Chem. 1989;54:5846.
-
-
Selected examples in synthesis:
- Dounay A. B., Overman L. E., Wrobelski A. D. J. Am. Chem. Soc. 2005;127:10186. - PubMed
- Lebsack A. D., Link J. T., Overman L. E., Stearns B. A. J. Am. Chem. Soc. 2002;124:9008. - PubMed
- Fox M. E., Li C., Marino Jr J. P., Overman L. E. J. Am. Chem. Soc. 1999;121:5467.
- Matsuura T., Overman L. E., Poon D. J. J. Am. Chem. Soc. 1998;120:6500.
-
-
- Salinosporamide A: Felig R. H., Buchanan G. O., Mincer T. J., Kauffman C. A., Jensen P. R., Fenical W., Angew. Chem., Int. Ed., 2003, 42 , 355 . - PubMed
- cylindricine C: Li C. P., Blackman A. J., Aust. J. Chem., 1994, 47 , 1355 .
- cephalotaxine: Powell R. G., Weisleder D., Smith Jr C. R., Wolff I. A., Tetrahedron Lett., 1969, 10 , 4081 .
-
-
. For reviews, see:
- Tsutsui H., Narasaka K. Chem. Lett. 1999;28:45.
- Tsutsui H., Kitamura M., Narasaka K. Bull. Chem. Soc. Jpn. 2002;75:1451.
- Kitamura M., Narasaka K. Chem. Rec. 2002;2:268. - PubMed
- Narasaka K., Kitamura M. Eur. J. Org. Chem. 2005:4505.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources