Development of a facile antibody-drug conjugate platform for increased stability and homogeneity
- PMID: 28451344
- PMCID: PMC5369337
- DOI: 10.1039/c6sc05149a
Development of a facile antibody-drug conjugate platform for increased stability and homogeneity
Abstract
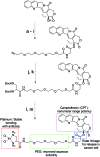
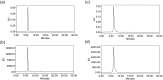
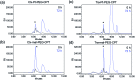
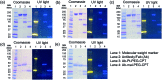
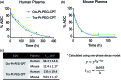
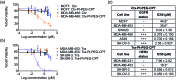
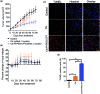
Despite the advances in the design of antibody-drug conjugates (ADCs), the search is still ongoing for novel approaches that lead to increased stability and homogeneity of the ADCs. We report, for the first time, an ADC platform technology using a platinum(ii)-based linker that can re-bridge the inter-chain cysteines in the antibody, post-reduction. The strong platinum-sulfur interaction improves the stability of the ADC when compared with a standard maleimide-linked ADC thereby reducing the linker-drug exchange with albumin significantly. Moreover, due to the precise conserved locations of cysteines, both homogeneity and site-specificity are simultaneously achieved. Additionally, we demonstrate that our ADCs exhibit increased anticancer efficacy in vitro and in vivo. The Pt-based ADCs can emerge as a simple and exciting proposition to address the limitations of the current ADC linker technologies.
Figures




Similar articles
-
Antibody-Drug Conjugates (ADCs) Derived from Interchain Cysteine Cross-Linking Demonstrate Improved Homogeneity and Other Pharmacological Properties over Conventional Heterogeneous ADCs.Mol Pharm. 2015 Nov 2;12(11):3986-98. doi: 10.1021/acs.molpharmaceut.5b00432. Epub 2015 Oct 2. Mol Pharm. 2015. PMID: 26393951 Free PMC article.
-
The Properties of Cysteine-Conjugated Antibody-Drug Conjugates Are Impacted by the IgG Subclass.AAPS J. 2018 Sep 25;20(6):103. doi: 10.1208/s12248-018-0263-0. AAPS J. 2018. PMID: 30255287
-
In Vivo Characterization of Platinum(II)-Based Linker Technology for the Development of Antibody-Drug Conjugates: Taking Advantage of Dual Labeling with 195mPt and 89Zr.J Nucl Med. 2018 Jul;59(7):1146-1151. doi: 10.2967/jnumed.117.206672. Epub 2018 Mar 1. J Nucl Med. 2018. PMID: 29496986
-
[Novel Chemical Linkers for Next-generation Antibody-drug Conjugates(ADCs)].Yakugaku Zasshi. 2019;139(2):209-219. doi: 10.1248/yakushi.18-00169-3. Yakugaku Zasshi. 2019. PMID: 30713230 Review. Japanese.
-
Current ADC Linker Chemistry.Pharm Res. 2015 Nov;32(11):3526-40. doi: 10.1007/s11095-015-1657-7. Epub 2015 Mar 11. Pharm Res. 2015. PMID: 25759187 Free PMC article. Review.
Cited by
-
The Evolving Landscape of Antibody-Drug Conjugates: In Depth Analysis of Recent Research Progress.Bioconjug Chem. 2023 Nov 15;34(11):1951-2000. doi: 10.1021/acs.bioconjchem.3c00374. Epub 2023 Oct 11. Bioconjug Chem. 2023. PMID: 37821099 Free PMC article. Review.
-
Monomethyl Auristatin E Grafted-Liposomes to Target Prostate Tumor Cell Lines.Int J Mol Sci. 2021 Apr 15;22(8):4103. doi: 10.3390/ijms22084103. Int J Mol Sci. 2021. PMID: 33921088 Free PMC article.
-
Monodisperse polysarcosine-based highly-loaded antibody-drug conjugates.Chem Sci. 2019 Mar 6;10(14):4048-4053. doi: 10.1039/c9sc00285e. eCollection 2019 Apr 14. Chem Sci. 2019. PMID: 31015945 Free PMC article.
-
Payload diversification: a key step in the development of antibody-drug conjugates.J Hematol Oncol. 2023 Jan 17;16(1):3. doi: 10.1186/s13045-022-01397-y. J Hematol Oncol. 2023. PMID: 36650546 Free PMC article. Review.
-
Redox-Responsive Dual Chemophotothermal Therapeutic Nanomedicine for Imaging-Guided Combinational Therapy.J Mater Chem B. 2018 Sep 7;6(33):5362-5367. doi: 10.1039/C8TB01360H. Epub 2018 Jul 23. J Mater Chem B. 2018. PMID: 30931124 Free PMC article.
References
-
- de Goeij B. E., Lambert J. M. Curr. Opin. Immunol. 2016;40:14–23. - PubMed
-
- Senter P. D., Sievers E. L. Nat. Biotechnol. 2012;30:631–637. - PubMed
-
- Lambert J. M., Chari R. V. J. J. Med. Chem. 2014;57:6949–6964. - PubMed
-
- Kim M. T.-J., Chen Y., Marhoul J., Jacobson F. Bioconjugate Chem. 2014;25:1223–1232. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

