Cobalt catalyzed carbonylation of unactivated C(sp3)-H bonds
- PMID: 28451350
- PMCID: PMC5369400
- DOI: 10.1039/c6sc05026c
Cobalt catalyzed carbonylation of unactivated C(sp3)-H bonds
Abstract
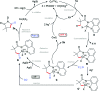
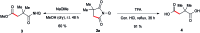
A general efficient regioselective cobalt catalyzed carbonylation of unactivated C(sp3)-H bonds of aliphatic amides was demonstrated using atmospheric (1-2 atm) carbon monoxide as a C1 source. This straightforward approach provides access to α-spiral succinimide regioselectively in a good yield. Cobalt catalyzed sp3 C-H bond carbonylation is reported for the first time including the functionalization of (β)-C-H bonds of α-1°, 2°, 3° carbons and even internal (β)-C-H bonds. Our initial mechanistic investigation reveals that the C-H activation step is irreversible and will possibly be the rate determining step.
Figures




References
-
- Beller M., Catalytic carbonylation reactions, topic in organometallic Chemistry, Springer, 2006, pp. 1–283.
-
- Gadge S. T., Gautam P., Bhanage B. M., Chem. Rec., 2016, 16 , 835 –856 , and references therein . - PubMed
-
- Murahashi S. J. Am. Chem. Soc. 1955;77:6403–6404.
-
-
Selected recent reviews see:
- Zhu R.-Y., Farmer M. E., Chen Y.-Q., Yu J. Q. Angew. Chem., Int. Ed. 2016;55:10578–10599. - PMC - PubMed
- Gao K., Yoshikai N. Acc. Chem. Res. 2014;47:1208. - PubMed
- Wu X.-F., Neumann H., Beller M. ChemSusChem. 2013;6:229–241. - PubMed
- Wu X.-F., Neumann H., Beller M. Chem. Rev. 2013;113:1–35. - PubMed
- Colby D. A., Bergmann R. G., Ellman J. A. Chem. Rev. 2010;110:624–655. - PMC - PubMed
- Brennuführer A., Neumann H., Beller M. ChemCatChem. 2009;1:28–41.
-
-
- Li H., Li B.-J., Shi Z.-J. Catal. Sci. Technol. 2011;1:191–206.
- Crabtree R. H. J. Organomet. Chem. 2004;689:4083–4091.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

