Plasmonic labeling of subcellular compartments in cancer cells: multiplexing with fine-tuned gold and silver nanoshells
- PMID: 28451372
- PMCID: PMC5380877
- DOI: 10.1039/c6sc04127b
Plasmonic labeling of subcellular compartments in cancer cells: multiplexing with fine-tuned gold and silver nanoshells
Abstract
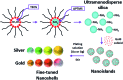
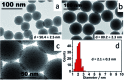
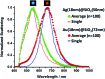
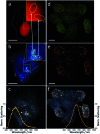
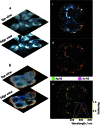
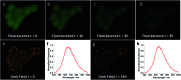
Fine-tuned gold and silver nanoshells were produced via an entirely reformulated synthesis. The new method yielded ultramonodisperse samples, with polydispersity indexes (PI) as low as 0.02 and narrow extinction bands suited for multiplex analysis. A library of nanoshell samples with localized surface plasmon resonances (LSPR) spanning across the visible range was synthesized. Hyperspectral analysis revealed that the average scattering spectrum of 100 nanoshells matched closely to the spectrum of a single nanoshell, indicating an unprecedented low level of nanoparticle-to-nanoparticle variation for this type of system. A cell labeling experiment, targeting different subcellular compartments in MCF-7 human breast cancer cells, demonstrated that these monodisperse nanoparticles can be used as a multiplex platform for single cell analysis at the intracellular and extracellular level. Antibody-coated gold nanoshells targeted the plasma membrane, while silver nanoshells coated with a nuclear localization signal (NLS) targeted the nuclear membrane. A fluorescence counterstaining experiment, as well as single cell hyperspectral microscopy showed the excellent selectivity and specificity of each type of nanoparticle for its designed subcellular compartment. A time-lapse photodegradation experiment confirmed the enhanced stability of the nanoshells over fluorescent labeling and their capabilities for long-term live cell imaging.
Figures


References
-
- Brito-Silva A. M., de Araujo C. B., Brayner F. A., Santos S. S., Galembeck A., Milet E. R. Polym. Eng. Sci. 2010;50:2350–2355.
-
- Brito-Silva A. M., Sobral-Filho R. G., Mejía H. A., Wang Y.-H., Wang P., Machado G., Falcão-Filho E. L., de Araújo C. B., Brolo A. G. J. Phys. Chem. C. 2014;118:18372–18376.
-
- Choi Y., Park Y., Kang T., Lee L. P. Nat. Nanotechnol. 2009;4:742–746. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

