Rare Complications of Cervical Spine Surgery: Pseudomeningocoele
- PMID: 28451481
- PMCID: PMC5400191
- DOI: 10.1177/2192568216687769
Rare Complications of Cervical Spine Surgery: Pseudomeningocoele
Abstract
Study design: This study was a retrospective, multicenter cohort study.
Objectives: Rare complications of cervical spine surgery are inherently difficult to investigate. Pseudomeningocoele (PMC), an abnormal collection of cerebrospinal fluid that communicates with the subarachnoid space, is one such complication. In order to evaluate and better understand the incidence, presentation, treatment, and outcome of PMC following cervical spine surgery, we conducted a multicenter study to pool our collective experience.
Methods: This study was a retrospective, multicenter cohort study of patients who underwent cervical spine surgery at any level(s) from C2 to C7, inclusive; were over 18 years of age; and experienced a postoperative PMC.
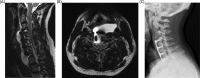
Results: Thirteen patients (0.08%) developed a postoperative PMC, 6 (46.2%) of whom were female. They had an average age of 48.2 years and stayed in hospital a mean of 11.2 days. Three patients were current smokers, 3 previous smokers, 5 had never smoked, and 2 had unknown smoking status. The majority, 10 (76.9%), were associated with posterior surgery, whereas 3 (23.1%) occurred after an anterior procedure. Myelopathy was the most common indication for operations that were complicated by PMC (46%). Seven patients (53%) required a surgical procedure to address the PMC, whereas the remaining 6 were treated conservatively. All PMCs ultimately resolved or were successfully treated with no residual effects.
Conclusions: PMC is a rare complication of cervical surgery with an incidence of less than 0.1%. They prolong hospital stay. PMCs occurred more frequently in association with posterior approaches. Approximately half of PMCs required surgery and all ultimately resolved without residual neurologic or other long-term effects.
Keywords: cervical spine; multicenter; pseudomeningocoele; retrospective.
Conflict of interest statement
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Tamir Ailon reports grants from AOSpine North America, during the conduct of the study; Justin S. Smith reports grants from AOSpine North America during the conduct of the study, personal fees from Biomet, personal fees from Nuvasive, personal fees from Cerapedics, personal fees from K2M, personal fees and other from DePuy, personal fees from Medtronic, outside the submitted work; Zachary A. Smith reports grants from AOSpine North America during the conduct of the study; Wellington K. Hsu reports grants from AOSpine North America during the conduct of the study, personal fees from Medtronic, personal fees from Stryker, personal fees from Bacterin, personal fees from Graftys, personal fees from Ceramtec, personal fees from Relievant, personal fees from Bioventus, personal fees from Globus, personal fees from SpineSmith, outside the submitted work; Michael G. Fehlings reports grants from AOSpine North America during the conduct of the study; David E. Fish reports grants from AOSpine North America during the conduct of the study; Jeffrey C. Wang reports grants from AOSpine North America during the conduct of the study; Alan S. Hilibrand reports grants from AOSpine North America during the conduct of the study, other from Amedica, Vertiflex, Benvenue, Lifespine, Paradigm Spine, PSD, Spinal Ventures, outside the submitted work, and in addition, Dr. Hilibrand has a patent Aesculap, Amedica, Biomet, Stryker, Alphatec, with royalties paid; Praveen V. Mummaneni reports grants from AOSpine North America during the conduct of the study, other from Depuy Spine, grants and other from AOSpine, other from Globus, other from Springer Publishers, other from Thieme Publishers, other from Taylor and Francis Publishers, other from Spincity/ISD, outside the submitted work; Dean Chou reports grants from AOSpine North America during the conduct of the study, other from Globus, other from Medtronic, other from Orthofix, outside the submitted work; Vincent C. Traynelis reports grants from AOSpine North America during the conduct of the study, and Medtronic - Royalties and Consultant Globus - Institutional Fellowship Support; Thomas E. Mroz reports other from AO Spine, grants from AOSpine North America during the conduct of the study, personal fees from Stryker, personal fees from Ceramtec, other from Pearl Diver, outside the submitted work; Zorica Buser reports grants from AOSpine North America during the conduct of the study; Elizabeth L. Lord reports grants from AOSpine North America during the conduct of the study; Eric M. Massicotte reports grants from AOSpine North America during the conduct of the study, grants from Medtronic, Depuy-Synthes Spine Canada, personal fees from Watermark consulting, grants from AOSpine North America, nonfinancial support from AOSpine North America, outside the submitted work; Arjun S. Sebastian reports grants from AOSpine North America during the conduct of the study; Khoi D. Than reports grants from AOSpine North America during the conduct of the study; Michael P. Steinmetz reports grants from AOSpine North America during the conduct of the study; Gabriel A. Smith reports grants from AOSpine North America during the conduct of the study; Jonathan Pace reports grants from AOSpine North America during the conduct of the study; K. Daniel Riew reports personal fees from AOSpine International, other from Global Spine Journal, other from Spine Journal, other from Neurosurgery, personal fees from Multiple Entities for defense, plantiff, grants from AOSpine, grants from Cerapedics, grants from Medtronic, personal fees from AOSpine, personal fees from NASS, personal fees from Biomet, personal fees from Medtronic, nonfinancial support from Broadwater, outside the submitted work; and Christopher Shaffrey reports grants from AOSpine North America during the conduct of the study, personal fees from Biomet, personal fees from Medtronic, from Nuvasive, personal fees from K2M, personal fees from Stryker, outside the submitted work, and Editorial Board Spine, Spinal Deformity and Neurosurgery; Hani R. Malone reports grants from AOSpine North America during the conduct of the study; Adam S. Kanter reports grants from AOSpine North America during the conduct of the study; Samuel K. Cho reports grants from AOSpine North America during the conduct of the study; grants from OREF, personal fees from Stryker, personal fees from Medtronic, personal fees from DePuy Synthes, outside the submitted work; Ra'Kerry K. Rahman reports grants from AOSpine North America during the conduct of the study; in addition, Dr. Rahman has a patent Deformity System & Pedicle Screws pending. Paul M. Arnold reports grants from AOSpine North America during the conduct of the study; other from Z-Plasty, other from Medtronic Sofamore Danek, other from Stryker Spine, other from FzioMed, other from AOSpine North America, other from Life Spine, other from Integra Life, other from Spine Wave, other from MIEMS, other from Cerapedics, other from AOSpine North America, outside the submitted work.
Figures
References
-
- Jain NK, Dao K, Ortiz AO. Radiologic evaluation and management of postoperative spine paraspinal fluid collections. Neuroimaging Clin N Am. 2014;24:375–389. doi:10.1016/j.nic.2014.01.001. - PubMed
-
- Shapiro SA, Scully T. Closed continuous drainage of cerebrospinal fluid via a lumbar subarachnoid catheter for treatment or prevention of cranial/spinal cerebrospinal fluid fistula. Neurosurgery. 1992;30:241–245. - PubMed
-
- Findler G, Sahar A, Beller AJ. Continuous lumbar drainage of cerebrospinal fluid in neurosurgical patients. Surg Neurol. 1977;8:455–457. - PubMed
-
- Hanakita J, Kinuta Y, Suzuki T. Spinal cord compression due to postoperative cervical pseudomeningocele. Neurosurgery. 1985;17:317–319. - PubMed
-
- Helle TL, Conley FK. Postoperative cervical pseudomeningocele as a cause of delayed myelopathy. Neurosurgery. 1981;9:314–316. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

