Factor VIIIa-mimetic cofactor activity of a bispecific antibody to factors IX/IXa and X/Xa, emicizumab, depends on its ability to bridge the antigens
- PMID: 28451690
- PMCID: PMC6292136
- DOI: 10.1160/TH17-01-0030
Factor VIIIa-mimetic cofactor activity of a bispecific antibody to factors IX/IXa and X/Xa, emicizumab, depends on its ability to bridge the antigens
Abstract
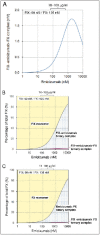
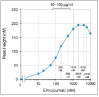
Emicizumab, a humanised bispecific antibody recognising factors (F) IX/IXa and X/Xa, can accelerate FIXa-catalysed FX activation by bridging FIXa and FX in a manner similar to FVIIIa. However, details of the emicizumab-antigen interactions have not been reported so far. In this study, we first showed by surface plasmon resonance analysis that emicizumab bound FIX, FIXa, FX, and FXa with moderate affinities (KD = 1.58, 1.52, 1.85, and 0.978 µM, respectively). We next showed by immunoblotting analysis that emicizumab recognised the antigens' epidermal growth factor (EGF)-like domains. We then performed KD-based simulation of equilibrium states in plasma for quantitatively predicting the ways that emicizumab would interact with the antigens. The simulation predicted that only a small part of plasma FIX, FX, and emicizumab would form antigen-bridging FIX-emicizumab-FX ternary complex, of which concentration would form a bell-shaped relationship with emicizumab concentration. The bell-shaped concentration dependency was reproduced by plasma thrombin generation assays, suggesting that the plasma concentration of the ternary complex would correlate with emicizumab's cofactor activity. The simulation also predicted that at 10.0-100 µg/ml of emicizumab-levels shown in a previous study to be clinically effective-the majority of plasma FIX, FX, and emicizumab would exist as monomers. In conclusion, emicizumab binds FIX/FIXa and FX/FXa with micromolar affinities at their EGF-like domains. The KD-based simulation predicted that the antigen-bridging ternary complex formed in circulating plasma would correlate with emicizumab's cofactor activity, and the majority of FIX and FX would be free and available for other coagulation reactions.
Keywords: Coagulation factors; drug design; factor VIII; haemophilia therapy.
Conflict of interest statement
Figures

References
-
- Srivastava A, Brewer AK, Mauser-Bunschoten EP et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1–e47.. - PubMed
-
- Peyvandi F, Garagiola I, Young G. The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet. 2016;388:187–197. - PubMed
-
- Leissinger CA. Advances in the clinical management of inhibitors in hemophilia A and B. Semin Hematol. 2016;53:20–27. - PubMed
-
- Kitazawa T, Igawa T, Sampei Z et al. A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia A model. Nat Med. 2012;18:1570–1574. - PubMed
-
- Lenting PJ, Donath MJ, van Mourik JA et al. Identification of a binding site for blood coagulation factor IXa on the light chain of human factor VIII. J Biol Chem. 1994;269:7150–7155. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

