Identification and management of patients at increased risk of osteoporotic fracture: outcomes of an ESCEO expert consensus meeting
- PMID: 28451733
- PMCID: PMC5483332
- DOI: 10.1007/s00198-017-4009-0
Identification and management of patients at increased risk of osteoporotic fracture: outcomes of an ESCEO expert consensus meeting
Erratum in
-
Erratum to: Identification and management of patients at increased risk of osteoporotic fracture: outcomes of an ESCEO expert consensus meeting.Osteoporos Int. 2017 Nov;28(11):3285-3286. doi: 10.1007/s00198-017-4161-6. Osteoporos Int. 2017. PMID: 28785979 Free PMC article. No abstract available.
Abstract
Osteoporosis represents a significant and increasing healthcare burden in Europe, but most patients at increased risk of fracture do not receive medication, resulting in a large treatment gap. Identification of patients who are at particularly high risk will help clinicians target appropriate treatment more precisely and cost-effectively, and should be the focus of future research.
Introduction: The purpose of the study was to review data on the identification and treatment of patients with osteoporosis at increased risk of fracture.
Methods: A working group convened by the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis met to review current data on the epidemiology and burden of osteoporosis and the patterns of medical management throughout Europe.
Results: In Europe in 2010, the cost of managing osteoporosis was estimated at €37 billion and notably the costs of treatment and long-term care of patients with fractures were considerably higher than the costs for pharmacological prevention. Despite the availability of effective treatments, the uptake of osteoporosis therapy is low and declining, in particular for secondary fracture prevention where the risk of a subsequent fracture following a first fracture is high. Consequently, there is a significant treatment gap between those who would benefit from treatment and those who receive it, which urgently needs to be addressed so that the burden of disease can be reduced.
Conclusions: Implementation of global fracture prevention strategies is a critical need. Future research should focus on identifying specific risk factors for imminent fractures, periods of high fracture risk, patients who are at increased risk of fracture and therapies that are most suited to such high-risk patients and optimal implementation strategies in primary, secondary and tertiary care.
Keywords: Fracture risk; Healthcare burden; Management; Osteoporosis; Secondary prevention; Treatment gap.
Conflict of interest statement
JAK reports grants from Amgen, Eli Lilly and Radius Health; non-financial support from Medimaps, and Asahi; and other support from AgNovos outside the submitted work. JAK is the architect of FRAX but has no financial interest. CC has received consultancy, lecture fees and honoraria from Amgen, GlaxoSmithKline, Alliance for Better Bone Health, Merck Sharp & Dohme, Eli Lilly, Pfizer, Novartis, Servier, Medtronic and Roche. RR has received consulting fees or advisory board fees from Radius Health, Labatec, Danone and Nestlé. BA has institutional research contracts with Novartis and UCB outside of the submitted work. NMA-D has received support from the Prince Mutaib Chair for Biomarkers of Osteoporosis, Deanship of Scientific Research Chairs, King Saud University. MLB has received consultancy fees and grants from Alexion, Abiogen, Amgen, Eli Lilly and Shire. JC-A has received grants and/or advisory board fees from Amgen, Servier, Fresenius-VIFOR and Shire. BC has received consultancy fees, lecture fees and honorarium from Amgen, Eli Lilly, Expanscience, Ferring, Medtronic, Novartis, Roche Diagnostics and Servier. HPD has received lecture fees, consulting fees and/or advisory board fees from Amgen, Daiichi-Sankyo, Eli Lilly, Genericon, Kyphon, Merck Sharp & Dohme, Novartis, Nycomed, Servier and Sinapharm. SF has received grants or research support from Amgen and Merck Sharp & Dohme and consultancy fees from Amgen, Merck Sharp & Dohme, GlaxoSmithKline, Eli Lilly and UCB. PH has received grants, advisory board or speaker fees from Amgen, AstraZeneca, Eli Lilly, Exeltis, Daichii-Sankyo, Gedeon Richter, Meda, Merck Sharp & Dohme, Mylan, Novartis, Pfizer, Roche and UCB. NCH has received consultancy, lecture fees and honoraria from Alliance for Better Bone Health, Amgen, Merck Sharp & Dohme, Eli Lilly, Servier, Shire, UCB, Consilient Healthcare and Internis Pharma. MK has no conflicts of interest to declare. AK has received consulting and speaker fees from Agnovos, Amgen, Eli Lilly, Novartis, Novo Nordisk, Roche, Servier, Biomet and Dfine, Inc. EM is or has acted as a consultant, advisor, speaker and/or received research support from ActiveSignal, Amgen, Arthritis Research UK, AstraZeneca, Consilient Healthcare, EPSRC, GlaxoSmithKline, Hologic, I3 Innovus, Internis, the International Osteoporosis Foundation, Eli Lilly, the Medical Research Council, Medtronic, Merck, Novartis, Pfizer, Roche, Sanofi-Aventis, Servier, Synexus, Tethys, UCB, Unilever and Warner Chilcott. SM has served as a speaker for Abiogen, Amgen, Diasorin, Eli Lilly, Italfarmaco, Fujii, Merck Sharp & Dohme and Takeda and on advisory boards for Amgen and Eli Lilly. TT has received advisory board or speaker fees from Amgen, Chugai/Roche, Expanscience, Genévrier, GlaxoSmithKline, HAC-Pharma, Eli Lilly, Medac, Merck Sharp & Dohme, Novartis, Teva and UCB and research grants or investigator fees from Amgen, Bone Therapeutics, Chugai/Roche, LCA, Merck Sharp & Dohme, Novartis, Pfizer and UCB. J-YR has received advisory board or speaker fees from Asahi-Kasei, Eli Lilly, IBSA-Genévrier, Nycomed-Takeda, PharmEvo, Radius Health, Roche, Servier, UCB, Will Pharma and Zodiac.
Figures

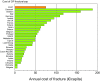




References
-
- Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jonsson B, Kanis JA. Osteoporosis in the European Union: medical management, epidemiology and economic burden: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch Osteoporos. 2013;8:136. doi: 10.1007/s11657-013-0136-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

