Prospective Clinical and Pharmacological Evaluation of the Delcath System's Second-Generation (GEN2) Hemofiltration System in Patients Undergoing Percutaneous Hepatic Perfusion with Melphalan
- PMID: 28451811
- PMCID: PMC5554291
- DOI: 10.1007/s00270-017-1630-4
Prospective Clinical and Pharmacological Evaluation of the Delcath System's Second-Generation (GEN2) Hemofiltration System in Patients Undergoing Percutaneous Hepatic Perfusion with Melphalan
Erratum in
-
Erratum to: Prospective Clinical and Pharmacological Evaluation of the Delcath System's Second-Generation (GEN2) Hemofiltration System in Patients Undergoing Percutaneous Hepatic Perfusion with Melphalan.Cardiovasc Intervent Radiol. 2017 Dec;40(12):1966. doi: 10.1007/s00270-017-1766-2. Cardiovasc Intervent Radiol. 2017. PMID: 28840267 Free PMC article. No abstract available.
Abstract
Introduction: Percutaneous hepatic perfusion (PHP) with melphalan is an effective treatment for patients with hepatic metastases, but associated with high rates of bone marrow depression. To reduce systemic toxicity, improvements have been made to the filtration system. In pre-clinical studies, the Delcath System's GEN2 filter was superior to the first-generation filters. In this clinical study, we analysed the pharmacokinetics and toxicity of PHP using the new GEN2 filter.
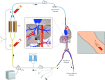
Methods and materials: Starting February 2014, two prospective phase II studies were initiated in patients with hepatic metastases from ocular melanoma or colorectal cancer. In 10 PHP procedures performed in the first 7 enrolled patients, blood samples were obtained to determine filter efficiency and systemic drug exposure. PHP was performed with melphalan 3 mg/kg with a maximum of 220 mg. Complications were assessed according to CTCAE v4.03. Response was assessed according to RECIST 1.1.
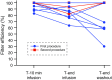
Results: Pharmacokinetic analysis of blood samples showed an overall filter efficiency of 86% (range 71.1-95.5%). The mean filter efficiency decreased from 95.4% 10 min after the start of melphalan infusion to 77.5% at the end of the procedure (p = 0.051). Bone marrow depression was seen after up to 80.0% of 10 procedures, but was self-limiting and mostly asymptomatic. No hypotension-related complications or procedure-related mortality occurred.
Conclusion: The GEN2 filter has a higher melphalan filter efficiency compared to the first-generation filters and a more consistent performance. PHP with the GEN2 filter appears to have an acceptable safety profile, but this needs further validation in larger studies.
Keywords: Cancer; Chemoembolisation; Colorectal cancer; Interventional oncology; Liver; Uveal melanoma.
Conflict of interest statement
Conflict of interest
Delcath Systems provided in-kind support and an educational grant for the conduct of the study. Three of the authors received travel fees to attend expert meetings sponsored by Delcath.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional and/or National Research Committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Figures

Similar articles
-
Safety of Percutaneous Hepatic Perfusion with Melphalan in Patients with Unresectable Liver Metastases from Ocular Melanoma Using the Delcath Systems' Second-Generation Hemofiltration System: A Prospective Non-randomized Phase II Trial.Cardiovasc Intervent Radiol. 2019 Jun;42(6):841-852. doi: 10.1007/s00270-019-02177-x. Epub 2019 Feb 14. Cardiovasc Intervent Radiol. 2019. PMID: 30767147 Free PMC article. Clinical Trial.
-
Safety and efficacy of chemosaturation in patients with primary and secondary liver tumors.J Cancer Res Clin Oncol. 2017 Oct;143(10):2113-2121. doi: 10.1007/s00432-017-2461-z. Epub 2017 Jun 20. J Cancer Res Clin Oncol. 2017. PMID: 28634727 Free PMC article.
-
Chemosaturation with percutaneous hepatic perfusion of melphalan for liver-dominant metastatic uveal melanoma: a single center experience.Cancer Imaging. 2019 May 30;19(1):31. doi: 10.1186/s40644-019-0218-4. Cancer Imaging. 2019. PMID: 31146793 Free PMC article.
-
Chemosaturation Percutaneous Hepatic Perfusion: A Systematic Review.Adv Ther. 2017 Jan;33(12):2122-2138. doi: 10.1007/s12325-016-0424-4. Epub 2016 Oct 31. Adv Ther. 2017. PMID: 27798773 Free PMC article.
-
The past decade of experience with isolated hepatic perfusion.Oncologist. 2004;9(6):653-64. doi: 10.1634/theoncologist.9-6-653. Oncologist. 2004. PMID: 15561809 Review.
Cited by
-
Unresectable Hepatic Metastasis of Uveal Melanoma: Hepatic Chemosaturation with High-Dose Melphalan-Long-Term Overall Survival Negatively Correlates with Tumor Burden.Radiol Res Pract. 2020 Sep 2;2020:5672048. doi: 10.1155/2020/5672048. eCollection 2020. Radiol Res Pract. 2020. PMID: 32934846 Free PMC article.
-
Endovascular Ion Exchange Chemofiltration Device Reduces Off-Target Doxorubicin Exposure in a Hepatic Intra-arterial Chemotherapy Model.Radiol Imaging Cancer. 2019 Sep 27;1(1):e190009. doi: 10.1148/rycan.2019190009. Radiol Imaging Cancer. 2019. PMID: 32300759 Free PMC article.
-
Percutaneous Hepatic Perfusion with Melphalan in Patients with Unresectable Ocular Melanoma Metastases Confined to the Liver: A Prospective Phase II Study.Ann Surg Oncol. 2021 Feb;28(2):1130-1141. doi: 10.1245/s10434-020-08741-x. Epub 2020 Aug 5. Ann Surg Oncol. 2021. PMID: 32761328 Free PMC article. Clinical Trial.
-
[Anesthesiological and postinterventional management in percutaneous hepatic melphalan perfusion (chemosaturation)].Anaesthesiologie. 2023 Feb;72(2):113-120. doi: 10.1007/s00101-022-01235-3. Epub 2022 Dec 7. Anaesthesiologie. 2023. PMID: 36477906 Free PMC article. Review. German.
-
Successful application of chemosaturation with percutaneous hepatic perfusion in metastatic uveal melanoma patient progressing after systemic treatment options: a case report.Front Oncol. 2024 Apr 10;14:1355971. doi: 10.3389/fonc.2024.1355971. eCollection 2024. Front Oncol. 2024. PMID: 38660135 Free PMC article.
References
-
- Hughes MS, Zager J, Faries M, Alexander HR, Royal RE, Wood B, et al. Results of a randomized controlled multicenter phase III trial of percutaneous hepatic perfusion compared with best available care for patients with melanoma liver metastases. Ann Surg Oncol. 2016;23(4):1309–1319. doi: 10.1245/s10434-015-4968-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

