A breast cancer gene signature for indolent disease
- PMID: 28451965
- PMCID: PMC5487706
- DOI: 10.1007/s10549-017-4262-0
A breast cancer gene signature for indolent disease
Abstract
Purpose: Early-stage hormone-receptor positive breast cancer is treated with endocrine therapy and the recommended duration of these treatments has increased over time. While endocrine therapy is considered less of a burden to patients compared to chemotherapy, long-term adherence may be low due to potential adverse side effects as well as compliance fatigue. It is of high clinical utility to identify subgroups of breast cancer patients who may have excellent long-term survival without or with limited duration of endocrine therapy to aid in personalizing endocrine treatment.
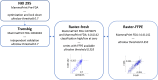
Methods: We describe a new ultralow risk threshold for the 70-gene signature (MammaPrint) that identifies a group of breast cancer patients with excellent 20 year, long-term survival prognosis. Tumors of these patients are referred to as "indolent breast cancer." We used patient series on which we previously established and assessed the 70-gene signature high-low risk threshold.
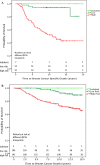
Results: In an independent validation cohort, we show that patients with indolent breast cancer had 100% breast cancer-specific survival at 15 years of follow-up.
Conclusions: Our data indicate that patients with indolent disease may be candidates for limited treatment with adjuvant endocrine therapy based on their very low risk of distant recurrences or death of breast cancer.
Keywords: Breast cancer; Indolent disease; MammaPrint; Ultralow threshold.
Conflict of interest statement
Authors (except CAD) are employed by Agendia (RB and LvtV part-time) and CD is a Consultant for Agendia, the commercial entity that markets the 70-gene signature as MammaPrint. LvtV and RB are Shareholders of Agendia and Named Inventors on the Patent for the 70-gene signature used in this study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures
References
-
- NCCN clinical practice guidelines in oncology for breast cancer version 1.2017 (2017). http://www.nccn.org/professionals/physician_gls/f_guidelines.asp
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

