Cloning and characterization of the Type I Baeyer-Villiger monooxygenase from Leptospira biflexa
- PMID: 28452041
- PMCID: PMC5407406
- DOI: 10.1186/s13568-017-0390-5
Cloning and characterization of the Type I Baeyer-Villiger monooxygenase from Leptospira biflexa
Abstract
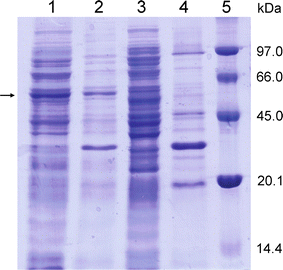
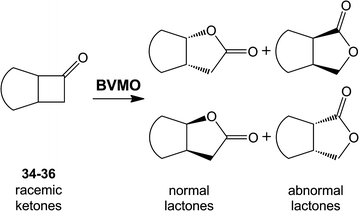
Baeyer-Villiger monooxygenases are recognized by their ability and high selectivity as oxidative biocatalysts for the generation of esters or lactones using ketones as starting materials. These enzymes represent valuable tools for biooxidative syntheses since they can catalyze reactions that otherwise involve strong oxidative reagents. In this work, we present a novel enzyme, the Type I Baeyer-Villiger monooxygenase from Leptospira biflexa. This protein is phylogenetically distant from other well-characterized BVMOs. In order to study this new enzyme, we cloned its gene, expressed it in Escherichia coli and characterized the substrate scope of the Baeyer-Villiger monooxygenase from L. biflexa as a whole-cell biocatalyst. For this purpose, we performed the screening of a collection of ketones with variable structures and sizes, namely acyclic ketones, aromatic ketones, cyclic ketones, and fused ketones. As a result, we observed that this biocatalyst readily oxidized linear- and branched- medium-chain ketones, alkyl levulinates and linear ketones with aromatic substituents with excellent regioselectivity. In addition, this enzyme catalyzed the oxidation of 2-substituted cycloketone derivatives but showed an unusual selection against substituents in positions 3 or 4 of the ring.
Keywords: Baeyer–Villiger monooxygenase; Biocatalysis; Ketones; Leptospira.
Figures


References
-
- Alexander AK, Biedermann D, Fink MJ, Mihovilovic MD, Mattes TE. Enantioselective oxidation by a cyclohexanone monooxygenase from the xenobiotic-degrading Polaromonas sp. strain JS666. J Mol Catal B Enzym. 2012;78:105–110. doi: 10.1016/j.molcatb.2012.03.002. - DOI
-
- Alphand V, Furstoss R. Microbiological transformations. 22. Microbiologically mediated Baeyer–Villiger reactions: a unique route to several bicyclic γ-lactones in high enantiomeric purity. J Org Chem. 1992;57:1306–1309. doi: 10.1021/jo00030a048. - DOI
-
- Alphand V, Mazzini C, Lebreton J, Furstoss R. A new microorganism for highly stereospecific Baeyer–Villiger oxidation of prochiral cyclobutanones. J Mol Catal B Enzym. 1998;5:219–221. doi: 10.1016/S1381-1177(98)00038-1. - DOI
-
- Beneventi E, Niero M, Motterle R, Fraaije MW, Bergantino E. Discovery of Baeyer–Villiger monooxygenases from photosynthetic eukaryotes. J Mol Catal B Enzym. 2013;98:145–154. doi: 10.1016/j.molcatb.2013.10.006. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

