Metabolomics Study of the Effects of Inflammation, Hypoxia, and High Glucose on Isolated Human Pancreatic Islets
- PMID: 28452488
- PMCID: PMC5557342
- DOI: 10.1021/acs.jproteome.7b00160
Metabolomics Study of the Effects of Inflammation, Hypoxia, and High Glucose on Isolated Human Pancreatic Islets
Abstract
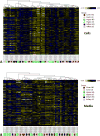
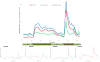
The transplantation of human pancreatic islets is a therapeutic possibility for a subset of type 1 diabetic patients who experience severe hypoglycemia. Pre- and post-transplantation loss in islet viability and function, however, is a major efficacy-limiting impediment. To investigate the effects of inflammation and hypoxia, the main obstacles hampering the survival and function of isolated, cultured, and transplanted islets, we conducted a comprehensive metabolomics evaluation of human islets in parallel with dynamic glucose-stimulated insulin release (GSIR) perifusion studies for functional evaluation. Metabolomics profiling of media and cell samples identified a total of 241 and 361 biochemicals, respectively. Metabolites that were altered in highly significant manner in both included, for example, kynurenine, kynurenate, citrulline, and mannitol/sorbitol under inflammation (all elevated) plus lactate (elevated) and N-formylmethionine (depressed) for hypoxia. Dynamic GSIR experiments, which capture both first- and second-phase insulin release, found severely depressed insulin-secretion under hypoxia, whereas elevated baseline and stimulated insulin-secretion was measured for islet exposed to the inflammatory cytokine cocktail (IL-1β, IFN-γ, and TNF-α). Because of the uniquely large changes observed in kynurenine and kynurenate, they might serve as potential biomarkers of islet inflammation, and indoleamine-2,3-dioxygenase on the corresponding pathway could be a worthwhile therapeutic target to dampen inflammatory effects.
Keywords: cytokines; human islets; hyperglycemia; hypoxia; insulin secretion; metabolomics; perifusion.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






Similar articles
-
Expression of the kynurenine pathway enzymes in the pancreatic islet cells. Activation by cytokines and glucolipotoxicity.Biochim Biophys Acta. 2015 May;1852(5):980-91. doi: 10.1016/j.bbadis.2015.02.001. Epub 2015 Feb 9. Biochim Biophys Acta. 2015. PMID: 25675848
-
Cytokine sensitivity of Langerhans' islets of diabetes-prone BB/OK rats under hypoglycemic conditions.Autoimmunity. 2003 Jun;36(4):211-9. doi: 10.1080/0891693031000116066. Autoimmunity. 2003. PMID: 14563014
-
Experimental evaluation and computational modeling of the effects of encapsulation on the time-profile of glucose-stimulated insulin release of pancreatic islets.Biomed Eng Online. 2015 Mar 28;14:28. doi: 10.1186/s12938-015-0021-9. Biomed Eng Online. 2015. PMID: 25889474 Free PMC article.
-
Protecting islet functional viability using mesenchymal stromal cells.Stem Cells Transl Med. 2021 May;10(5):674-680. doi: 10.1002/sctm.20-0466. Epub 2021 Feb 5. Stem Cells Transl Med. 2021. PMID: 33544449 Free PMC article. Review.
-
Formulation strategies to provide oxygen-release to contrast local hypoxia for transplanted islets.Eur J Pharm Biopharm. 2023 Jun;187:130-140. doi: 10.1016/j.ejpb.2023.04.015. Epub 2023 Apr 25. Eur J Pharm Biopharm. 2023. PMID: 37105362 Review.
Cited by
-
The Influence of Microenvironment on Survival of Intraportal Transplanted Islets.Front Immunol. 2022 Mar 28;13:849580. doi: 10.3389/fimmu.2022.849580. eCollection 2022. Front Immunol. 2022. PMID: 35418988 Free PMC article. Review.
-
Exploring new frontiers in type 1 diabetes through advanced mass-spectrometry-based molecular measurements.Trends Mol Med. 2024 Dec;30(12):1137-1151. doi: 10.1016/j.molmed.2024.07.009. Epub 2024 Aug 15. Trends Mol Med. 2024. PMID: 39152082 Review.
-
A novel approach to determine the critical survival threshold of cellular oxygen within spheroids via integrating live/dead cell imaging with oxygen modeling.Am J Physiol Cell Physiol. 2024 Apr 1;326(4):C1262-C1271. doi: 10.1152/ajpcell.00024.2024. Epub 2024 Mar 18. Am J Physiol Cell Physiol. 2024. PMID: 38497111 Free PMC article.
-
Layer-by-Layer Cerium Oxide Nanoparticle Coating for Antioxidant Protection of Encapsulated Beta Cells.Adv Healthc Mater. 2019 Jun;8(12):e1801493. doi: 10.1002/adhm.201801493. Epub 2019 Jan 11. Adv Healthc Mater. 2019. PMID: 30633854 Free PMC article.
-
Beneficial Effects of Two Marine Oxygen Carriers, M101 and M201, on Human Islet Quality in Hypoxic Culture Conditions.Cell Transplant. 2023 Jan-Dec;32:9636897231179642. doi: 10.1177/09636897231179642. Cell Transplant. 2023. PMID: 37318185 Free PMC article.
References
-
- Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nat. Rev. Immunol. 2004;4(4):259–268. - PubMed
-
- Fiorina P, Shapiro AM, Ricordi C, Secchi A. The clinical impact of islet transplantation. Am. J. Transplant. 2008;8(10):1990–1997. - PubMed
-
- Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hunsicker LG, Kaufman DB, Korsgren O, Larsen CP, Luo X, Markmann JF, Naji A, Oberholzer J, Posselt AM, Rickels MR, Ricordi C, Robien MA, Senior PA, Shapiro AM, Stock PG, Turgeon NA Clinical Islet Transplantation, C. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2016;39(7):1230–1240. - PMC - PubMed
-
- Shapiro AM, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat. Rev. Endocrinol. 2017;13(5):268–277. - PubMed
-
- Ricordi C, Goldstein JS, Balamurugan AN, Szot GL, Kin T, Liu C, Czarniecki CW, Barbaro B, Bridges ND, Cano J, Clarke WR, Eggerman TL, Hunsicker LG, Kaufman DB, Khan A, Lafontant DE, Linetsky E, Luo X, Markmann JF, Naji A, Korsgren O, Oberholzer J, Turgeon NA, Brandhorst D, Friberg AS, Lei J, Wang LJ, Wilhelm JJ, Willits J, Zhang X, Hering BJ, Posselt AM, Stock PG, Shapiro AM. National Institutes of Health-Sponsored Clinical Islet Transplantation Consortium Phase 3 Trial: Manufacture of a complex cellular product at eight processing facilities. Diabetes. 2016;65(11):3418–3428. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

