Donor-dependent variances of human adipose-derived stem cells in respect to the in-vitro endothelial cell differentiation capability
- PMID: 28452591
- PMCID: PMC5358701
- DOI: 10.1080/21623945.2016.1273299
Donor-dependent variances of human adipose-derived stem cells in respect to the in-vitro endothelial cell differentiation capability
Abstract
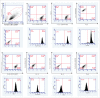
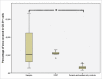
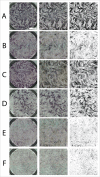
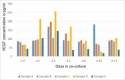
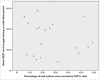
Human adipose-derived stem cells (ASC) have been shown to differentiate into mature adipocytes and to play an important role in creating the vasculature, necessary for white adipose tissue to function. To study the stimulatory capacity of ASC on endothelial progenitor cells we used a commercially available co-culture system (V2a - assay). ASC, isolated from lipoaspirates of 18 healthy patients, were co-cultured for 13 d on endothelial progenitor cells. Using anti CD31 immunostaining, cells that had undergone endothelial differentiation were quantified after the defined co-cultivation period. Endothelial cell differentiation was observed and demonstrated by an increase in area covered by CD31+ cells compared with less to no endothelial cell differentiation in negative and media-only controls. Enzyme-linked immunosorbent assay (ELISA) for vascular endothelial growth factor (VEGF) in supernatant medium collected during the co-cultivation period revealed elevated VEGF levels in the co-culture samples as compared with ASC cultures alone, whereas no increase in adiponectin was detected by ELISA. These findings help to provide further insights in the complex interplay of adipose derived cells and endothelial cells and to better understand the diversity of ASCs in respect of their stimulatory capacity to promote angiogenesis in vitro.
Keywords: adipose stem cell; angiogenesis; cell-cell interaction; endothelial cell; vasculogenesis.
Figures


References
-
- Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov 2010; 9:107-15; PMID:20118961; http://dx.doi.org/ 10.1038/nrd3055 - DOI - PubMed
-
- Jannson PA. Endothelial dysfunction in insulin resistance and type 2 diabetes. J Intern Med 2007; 262:173-83; http://dx.doi.org/ 10.1111/j.1365-2796.2007.01830.x - DOI - PubMed
-
- Strassburg S, Nienhueser H, Stark GB, Finkenzeller G, Torio-Padron N. Human adipose-derived stem cells enhance the angiogenic potential of endothelial progenitor cells, but not of human umbilical vein endothelial cells. Tissue Eng Part A 2013; 19:166-74; http://dx.doi.org/ 10.1089/ten.tea.2011.0699 - DOI - PubMed
-
- Alharbi Z, Almakadi S, Opländer C, Vogt M, Rennekampff HO, Pallua N, Intraoperative use of enriched collagen and elastin matrices with freshly isolated adipose-derived stem/stromal cells: a potential clinical approach for soft tissue reconstruction. 2014, BMC Surgery, 14:10; PMID:24555437; http://dx.doi.org/ 10.1186/1471-2482-14-10 - DOI - PMC - PubMed
-
- Tholpady SS, Llull R, Ogle RC, Rubin JP, Futrell JW, Katz AJ: Adipose tissue: stem cells and beyond. Clin Plast Surg 2006, 33(1):55-62; PMID:16427974; http://dx.doi.org/ 10.1016/j.cps.2005.08.004 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
