Estrogen Modulates Specific Life and Death Signals Induced by LH and hCG in Human Primary Granulosa Cells In Vitro
- PMID: 28452938
- PMCID: PMC5454839
- DOI: 10.3390/ijms18050926
Estrogen Modulates Specific Life and Death Signals Induced by LH and hCG in Human Primary Granulosa Cells In Vitro
Abstract
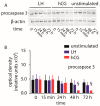
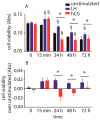
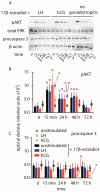
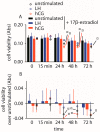
Luteinizing hormone (LH) and human chorionic gonadotropin (hCG) are glycoprotein hormones used for assisted reproduction acting on the same receptor (LHCGR) and mediating different intracellular signaling. We evaluated the pro- and anti-apoptotic effect of 100 pM LH or hCG, in the presence or in the absence of 200 pg/mL 17β-estradiol, in long-term, serum-starved human primary granulosa cells (hGLC) and a transfected granulosa cell line overexpressing LHCGR (hGL5/LHCGR). To this purpose, phospho-extracellular-regulated kinase 1/2 (pERK1/2), protein kinase B (pAKT), cAMP-responsive element binding protein (pCREB) activation and procaspase 3 cleavage were evaluated over three days by Western blotting, along with the expression of target genes by real-time PCR and cell viability by colorimetric assay. We found that LH induced predominant pERK1/2 and pAKT activation STARD1, CCND2 and anti-apoptotic XIAP gene expression, while hCG mediated more potent CREB phosphorylation, expression of CYP19A1 and procaspase 3 cleavage than LH. Cell treatment by LH is accompanied by increased (serum-starved) cell viability, while hCG decreased the number of viable cells. The hCG-specific, pro-apoptotic effect was blocked by a physiological dose of 17β-estradiol, resulting in pAKT activation, lack of procaspase 3 cleavage and increased cell viability. These results confirm that relatively high levels of steroidogenic pathway activation are linked to pro-apoptotic signals in vitro, which may be counteracted by other factors, i.e., estrogens.
Keywords: LH; apoptosis; gonadotropins; granulosa; hCG.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

