Protective Effect of Caffeic Acid Derivatives on tert-Butyl Hydroperoxide-Induced Oxidative Hepato-Toxicity and Mitochondrial Dysfunction in HepG2 Cells
- PMID: 28452956
- PMCID: PMC6154103
- DOI: 10.3390/molecules22050702
Protective Effect of Caffeic Acid Derivatives on tert-Butyl Hydroperoxide-Induced Oxidative Hepato-Toxicity and Mitochondrial Dysfunction in HepG2 Cells
Abstract
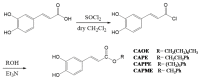
Oxidative stress results in structural and functional abnormalities in the liver and is thought to be a crucial factor in liver diseases. The aim of this study was to investigate the cytoprotective and antioxidant effects of caffeic acid (CA) derivatives on tert-butyl hydroperoxide (t-BHP)-induced oxidative stress in HepG2 cells. Nine CA derivatives were synthesized, including N-phenylethyl caffeamide (PECA), N-(3-florophen)methyl caffeamide (FMCA), N-(4-methoxy-phen)methyl caffeamide (MPMCA), N-heptyl caffeamide (HCA), N-octyl caffeamide (OCA), octyl caffeate (CAOE), phenpropyl caffeate (CAPPE), phenethyl caffeate (CAPE), and phenmethyl caffeate (CAPME). The results showed that CA and its derivatives significantly inhibited t-BHP-induced cell death of HepG2 cells. The rank order of potency of the CA derivatives for cytoprotection was CAOE > HCA > OCA > FMCA > CAPPE > CAPME > CAPE > PECA > MPMCA > CA. Their cytoprotective activity was associated with lipophilicity. The antioxidant effect of these compounds was supported by the reduction in the levels of thiobarbituric acid reactive substrates, a biomarker of lipid peroxidation, in HepG2 cells. Pre-treatment of CA derivatives significantly prevented the depletion of glutathione, the most important water-soluble antioxidant in hepatocytes. Pre-treatment of CA derivatives before t-BHP exposure maintained mitochondrial oxygen consumption rate and ATP content in the injured HepG2 cells. CA derivatives except OCA and HCA significantly suppressed t-BHP-induced hypoxia-inducible factor-1α (HIF-1α) protein level. In addition, all of these CA derivatives markedly increased the nuclear factor erythroid 2-related factor 2 (Nrf2) accumulation in the nucleus, indicating that their cytoprotection may be mediated by the activation of Nrf2. Our results suggest that CA derivatives might be a hepatoprotective agent against oxidative stress.
Keywords: antioxidant; caffeic acid derivatives; liver protection; tert-butyl hydroperoxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

