Nanoscale movements of cellulose microfibrils in primary cell walls
- PMID: 28452988
- PMCID: PMC5478883
- DOI: 10.1038/nplants.2017.56
Nanoscale movements of cellulose microfibrils in primary cell walls
Erratum in
-
Author Correction: Nanoscale movements of cellulose microfibrils in primary cell walls.Nat Plants. 2020 Dec;6(12):1504. doi: 10.1038/s41477-020-00821-w. Nat Plants. 2020. PMID: 33244172 No abstract available.
Abstract
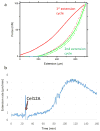
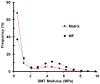
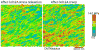
The growing plant cell wall is commonly considered to be a fibre-reinforced structure whose strength, extensibility and anisotropy depend on the orientation of crystalline cellulose microfibrils, their bonding to the polysaccharide matrix and matrix viscoelasticity1-4. Structural reinforcement of the wall by stiff cellulose microfibrils is central to contemporary models of plant growth, mechanics and meristem dynamics4-12. Although passive microfibril reorientation during wall extension has been inferred from theory and from bulk measurements13-15, nanometre-scale movements of individual microfibrils have not been directly observed. Here we combined nanometre-scale imaging of wet cell walls by atomic force microscopy (AFM) with a stretching device and endoglucanase treatment that induces wall stress relaxation and creep, mimicking wall behaviours during cell growth. Microfibril movements during forced mechanical extensions differ from those during creep of the enzymatically loosened wall. In addition to passive angular reorientation, we observed a diverse repertoire of microfibril movements that reveal the spatial scale of molecular connections between microfibrils. Our results show that wall loosening alters microfibril connectivity, enabling microfibril dynamics not seen during mechanical stretch. These insights into microfibril movements and connectivities need to be incorporated into refined models of plant cell wall structure, growth and morphogenesis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Comment in
-
Plant cell growth: Cellulose caught slipping.Nat Plants. 2017 Apr 28;3:17063. doi: 10.1038/nplants.2017.63. Nat Plants. 2017. PMID: 28452998 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

