Not just browsing: an animal that grazes phyllosphere microbes facilitates community heterogeneity
- PMID: 28452997
- PMCID: PMC5520039
- DOI: 10.1038/ismej.2017.52
Not just browsing: an animal that grazes phyllosphere microbes facilitates community heterogeneity
Abstract
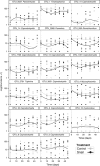
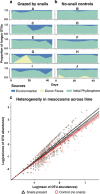
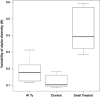
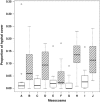
Although grazers have long been recognized as top-down architects of plant communities, animal roles in determining microbial community composition have seldom been examined, particularly in aboveground systems. To determine the extent to which an animal can shape microbial communities, we conducted a controlled mesocosm study in situ to see if introducing mycophageous tree snails changed phyllosphere fungal community composition relative to matched control mesocosms. Fungal community composition and change was determined by Illumina sequencing of DNA collected from leaf surfaces before snails were introduced, daily for 3 days and weekly for 6 weeks thereafter. Scanning electron microscopy was used to confirm that grazing had occurred, and we recorded 3.5 times more cover of fungal hyphae in control mesocosms compared with those containing snails. Snails do not appear to vector novel microbes and despite grazing, a significant proportion of the initial leaf phyllosphere persisted in the mesocosms. Within-mesocosm diversities of fungi were similar regardless of whether or not snails were added. The greatest differences between the snail-treated and control mesocosms was that grazed mesocosms showed greater infiltration of microbes that were not sampled when the experiment commenced and that the variance in fungal community composition (beta diversity) was greater between leaves in snail-treated mesocosms indicating increased community heterogeneity and ecosystem fragmentation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Beaver RA. (1989) Insect - Fungus relationships in the bark and ambrosia beetles. In: Wilding N, Collins NM, Hammond PM, Webber JF (eds). Insect - Fungus Interactions. Academic Press: New York, NY, USA, pp 121–143.
-
- Belyea LR, Lancaster J. (1999). Assembly rules within a contingent ecology. Oikos 86: 402–416.
-
- Berlow EL. (1999). Strong effects of weak interactions in ecological communities. Nature 398: 330–334.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

