PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease
- PMID: 28453187
- PMCID: PMC6478267
- DOI: 10.1002/14651858.CD011748.pub2
PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease
Update in
-
PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease.Cochrane Database Syst Rev. 2020 Oct 20;10(10):CD011748. doi: 10.1002/14651858.CD011748.pub3. Cochrane Database Syst Rev. 2020. PMID: 33078867 Free PMC article.
Abstract
Background: Despite the availability of effective drug therapies that reduce low-density lipoprotein (LDL)-cholesterol (LDL-C), cardiovascular disease (CVD) remains an important cause of mortality and morbidity. Therefore, additional LDL-C reduction may be warranted, especially for patients who are unresponsive to, or unable to take, existing LDL-C-reducing therapies. By inhibiting the proprotein convertase subtilisin/kexin type 9 (PCSK9) enzyme, monoclonal antibodies (PCSK9 inhibitors) may further reduce LDL-C, potentially reducing CVD risk as well.
Objectives: Primary To quantify short-term (24 weeks), medium-term (one year), and long-term (five years) effects of PCSK9 inhibitors on lipid parameters and on the incidence of CVD. Secondary To quantify the safety of PCSK9 inhibitors, with specific focus on the incidence of type 2 diabetes, cognitive function, and cancer. Additionally, to determine if specific patient subgroups were more or less likely to benefit from the use of PCSK9 inhibitors.
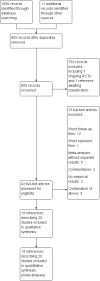
Search methods: We identified studies by systematically searching the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and Web of Science. We also searched Clinicaltrials.gov and the International Clinical Trials Registry Platform and screened the reference lists of included studies. We identified the studies included in this review through electronic literature searches conducted up to May 2016, and added three large trials published in March 2017.
Selection criteria: All parallel-group and factorial randomised controlled trials (RCTs) with a follow-up time of at least 24 weeks were eligible.
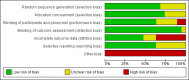
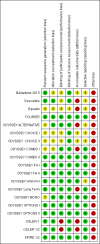
Data collection and analysis: Two review authors independently reviewed and extracted data. When data were available, we calculated pooled effect estimates.
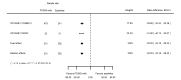
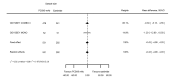
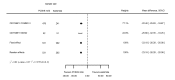
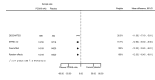
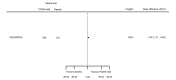
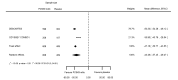
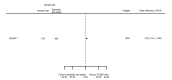
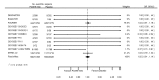
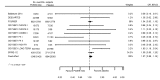
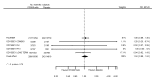
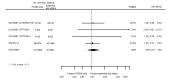
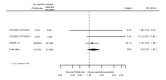
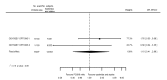
Main results: We included 20 studies with data on 67,237 participants (median age 61 years; range 52 to 64 years). Twelve trials randomised participants to alirocumab, three trials to bococizumab, one to RG7652, and four to evolocumab. Owing to the small number of trials using agents other than alirocumab, we did not differentiate between types of PCSK9 inhibitors used. We compared PCSK9 inhibitors with placebo (thirteen RCTs), ezetimibe (two RCTs) or ezetimibe and statins (five RCTs).Compared with placebo, PCSK9 inhibitors decreased LDL-C by 53.86% (95% confidence interval (CI) 58.64 to 49.08; eight studies; 4782 participants; GRADE: moderate) at 24 weeks; compared with ezetimibe, PCSK9 inhibitors decreased LDL-C by 30.20% (95% CI 34.18 to 26.23; two studies; 823 participants; GRADE: moderate), and compared with ezetimibe and statins, PCSK9 inhibitors decreased LDL-C by 39.20% (95% CI 56.15 to 22.26; five studies; 5376 participants; GRADE: moderate).Compared with placebo, PCSK9 inhibitors decreased the risk of CVD events, with a risk difference (RD) of 0.91% (odds ratio (OR) of 0.86, 95% CI 0.80 to 0.92; eight studies; 59,294 participants; GRADE: moderate). Compared with ezetimibe and statins, PCSK9 inhibitors appeared to have a stronger protective effect on CVD risk, although with considerable uncertainty (RD 1.06%, OR 0.45, 95% CI 0.27 to 0.75; three studies; 4770 participants; GRADE: very low). No data were available for the ezetimibe only comparison. Compared with placebo, PCSK9 probably had little or no effect on mortality (RD 0.03%, OR 1.02, 95% CI 0.91 to 1.14; 12 studies; 60,684 participants; GRADE: moderate). Compared with placebo, PCSK9 inhibitors increased the risk of any adverse events (RD 1.54%, OR 1.08, 95% CI 1.04 to 1.12; 13 studies; 54,204 participants; GRADE: low). Similar effects were observed for the comparison of ezetimibe and statins: RD 3.70%, OR 1.18, 95% CI 1.05 to 1.34; four studies; 5376 participants; GRADE: low. Clinical event data were unavailable for the ezetimibe only comparison.
Authors' conclusions: Over short-term to medium-term follow-up, PCSK9 inhibitors reduced LDL-C. Studies with medium-term follow-up time (longest median follow-up recorded was 26 months) reported that PCSK9 inhibitors (compared with placebo) decreased CVD risk but may have increased the risk of any adverse events (driven by SPIRE-1 and -2 trials). Available evidence suggests that PCSK9 inhibitor use probably leads to little or no difference in mortality. Evidence on relative efficacy and safety when PCSK9 inhibitors were compared with active treatments was of low to very low quality (GRADE); follow-up times were short and events were few. Large trials with longer follow-up are needed to evaluate PCSK9 inhibitors versus active treatments as well as placebo. Owing to the predominant inclusion of high-risk patients in these studies, applicability of results to primary prevention is limited. Finally, estimated risk differences indicate that PCSK9 inhibitors only modestly change absolute risks (often to less than 1%).
Conflict of interest statement
Amand F Schmidt: none known.
Lucy S Pearce: none known.
John T Wilkins: none known.
John P Overington: none known.
Aroon Hingorani is a member of the organisation committee of The Genetics of Subsequent Coronary Heart Disease Consortium and the Heart failure Molecular Epidemiology for Therapeutic Targets Consortium (HERMES) each comprising over 20 member cohorts. A number of Pharma companies have provided direct and in kind support for these initiatives, but AH is not a direct recipient of any of these funds.
Juan P Casas: none known.
Figures






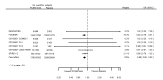
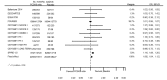
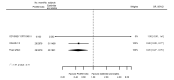
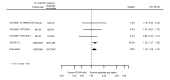




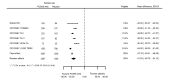

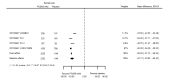





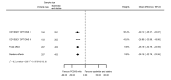

















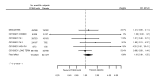
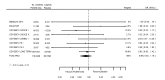
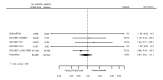

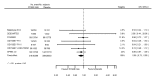
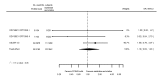
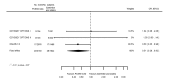

Comment in
-
Cochrane corner: PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease.Heart. 2018 Jul;104(13):1053-1055. doi: 10.1136/heartjnl-2017-312858. Epub 2018 Feb 14. Heart. 2018. PMID: 29444808 No abstract available.
References
References to studies included in this review
-
- Ballantyne CM, Neutel J, Cropp A, Duggan W, Wang EQ, Plowchalk D, et al. Results of bococizumab, a monoclonal antibody against proprotein convertase subtilisin/kexin type 9, from a randomized, placebo‐controlled, dose‐ranging study in statin‐treated subjects with hypercholesterolemia. American Journal of Cardiology 2015;115(9):1212‐21. - PubMed
-
- Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, et al. A 52‐week placebo‐controlled trial of evolocumab in hyperlipidemia. New England Journal of Medicine 2014;370:1809‐19. - PubMed
-
- Tingley W, Mosesova S, Baruch A, Davis JD, Budha N, Vilimovskij A, et al. Effects of RG7652, a monoclonal antibody against proprotein convertase Subtilisin/Kexin type 9, on LDL cholesterol in patients with coronary heart disease or high risk: results from the EQUATOR study. European Heart Journal. 2014:371.
-
- Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. New England Journal of Medicine 2017;online:1‐10. - PubMed
-
- Moriarty PM, Thompson PD, Cannon CP, Guyton JR, Bergeron J, Zieve FJ, et al. Efficacy and safety of alirocumab vs ezetimibe in statin‐intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. Journal of Clinical Lipidology 2015;9(6):758‐69. - PubMed
References to studies excluded from this review
-
- Baruch A, Peng K, Leabman M, Budha N, Luca D, Cowan KJ, et al. Effect of RG7652, a mAb against PCSK9, on apolipoprotein B, oxidized LDL, lipoprotein(A) and lipoprotein‐associated phospholipase a2 in healthy individuals with elevated LDL‐C. Circulation. 2013; Vol. 22.
-
- Cho L, Rocco M, Colquhoun D, Sullivan D, Rosenson RS, Dent R, et al. Clinical profile of statin intolerance in the phase 3 gauss‐2 study. Canadian Journal of Cardiology. 2014:S79. - PubMed
-
- Desai NR, Giugliano RP, Zhou J, Kohli P, Somaratne R, Hoffman E, et al. AMG 145, a monoclonal antibody against PCSK9, facilitates achievement of National Cholesterol Education Program‐Adult Treatment Panel III low‐density lipoprotein cholesterol goals among high‐risk patients: an analysis from the LAPLACE‐TIMI 57 trial (LDL‐C assessment with PCSK9 monoclonal antibody inhibition combined with statin thErapy‐thrombolysis in myocardial infarction 57). Journal of the American College of Cardiology 2014;63:430‐3. - PubMed
-
- Dias CS, Shaywitz AJ, Wasserman SM, Smith BP, Gao B, Stolman DS, et al. Effects of AMG 145 on low‐density lipoprotein cholesterol levels: results from 2 randomized, double‐blind, placebo‐controlled, ascending‐dose phase 1 studies in healthy volunteers and hypercholesterolemic subjects on statins. Journal of the American College of Cardiology 2012;60:1888‐98. - PubMed
-
- Dufour R, Moriarty PM, Genestin E, Sasiela WJ, Du Y, Ferrand AC, et al. Effect of REGN727/SAR236553 anti‐proprotein convertase subtilisin/kexin type 9 fully human monoclonal antibody in patients with elevated triglycerides/low high‐density lipoprotein cholesterol: data from three phase 2 studies (NCT:01266876; 01288469; 01288443). Circulation. 2012.
References to studies awaiting assessment
-
- Ridker PM, Tardif JC, Amarenco P, DUggan W, Glyn RJ, Jukema WJ, et al. Lipid‐reduction variability and antidrug‐antibody formation with bococizumab. New England Journal of Medicine 2017;online first:1‐10. - PubMed
References to ongoing studies
-
- NCT02729025. Effects of Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibition on Arterial Wall Inflamation Study in Patients With Elevated Lipoprotein(a) (Lp(a)). (ANITSCHKOW). https://clinicaltrials.gov/ct2/show/NCT02729025 (first received: March 8....
-
- NCT02207634. Evaluating PCSK9 Binding antiBody Influence oN coGnitive HeAlth in High cardiovascUlar Risk Subjects (EBBINGHAUS). https://clinicaltrials.gov/ct2/show/NCT02207634 (first received July 31,....
-
- NCT02392559. Trial Assessing Efficacy, Safety and Tolerability of PCSK9 Inhibition in Paediatric Subjects With Genetic LDL Disorders (HAUSER‐RCT). https://clinicaltrials.gov/ct2/show/NCT02392559 (first received February....
-
- NCT02833844. Safety, Tolerability & Efficacy on LDL‐C of Evolocumab in Subjects With HIV & Hyperlipidemia/Mixed Dyslipidemia. https://clinicaltrials.gov/ct2/show/NCT02833844 (first received June 13,....
-
- NCT02642159. Efficacy and Safety of Alirocumab Versus Usual Care on Top of Maximally Tolerated Statin Therapy in Patients With Type 2 Diabetes and Mixed Dyslipidemia (ODYSSEY DM‐Dyslipidemia). https://clinicaltrials.gov/ct2/show/NCT02642159 (first received December....
Additional references
-
- Abifadel M, Varret M, Rabès JP, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nature Genetics 2013;34(2):154‐6. - PubMed
-
- Benn M, Nordestgaard BG, Grande P, Schnohr P, Tybjaerg‐Hansen A. PCSK9 R46L, low‐density lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta‐analyses. Journal of the American College of Cardiology 2010;55(22):2833‐42. - PubMed
-
- Benn M, Watts GF, Tybjaerg‐Hansen A, Nordestgaard BG. Familial hypercholesterolemia in the Danish general population: prevalence, coronary artery disease, and cholesterol‐lowering medication. Journal of Clinical Endocrinology and Metabolism 2012;97(11):3956‐64. - PubMed
-
- Bradburn MJ, Deeks JJ, Berlin JA, Localio AR. Much ado about nothing: a comparison of the performance of meta‐analytical methods with rare events. Statistics in Medicine 2007;26(1):53‐77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

