Enantioselective Intermolecular Addition of Aliphatic Amines to Acyclic Dienes with a Pd-PHOX Catalyst
- PMID: 28453290
- PMCID: PMC5937019
- DOI: 10.1021/jacs.7b03480
Enantioselective Intermolecular Addition of Aliphatic Amines to Acyclic Dienes with a Pd-PHOX Catalyst
Abstract
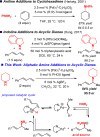
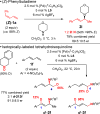
We report a method for the catalytic, enantioselective intermolecular addition of aliphatic amines to acyclic 1,3-dienes. In most cases, reactions proceed efficiently at or below room temperature in the presence of 5 mol % of a Pd catalyst bearing a PHOX ligand, generating allylic amines in up to 97:3 er. The presence of an electron-deficient phosphine within the ligand not only leads to a more active catalyst but also is critical for achieving high site selectivity in the transformation.
Figures
References
-
-
For reviews, see: Reznichenko AL, Nawara-Hultzsch AJ, Hultzsch KC. Top. Curr. Chem. 2013;343:191.Huang L, Arndt M, Gooßen K, Heydt H, Gooßen LJ. Chem. Rev. 2015;115:2596.Coman SM, Parvulescu VI. Org. Process Res. Dev. 2015;19:1327.
-
-
-
For reviews on Cu-catalyzed umpolung approaches to hydroamination of unsaturated hydrocarbons, see: Hirano K, Miura M. Pure Appl. Chem. 2014;86:291.Pirnot MT, Wang Y-M, Buchwald SL. Angew. Chem. Int. Ed. 2016;55:48.
-
-
- Löber O, Kawatsura M, Hartwig JF. J. Am. Chem. Soc. 2001;123:4366. - PubMed
-
-
For enantioselective Pd-catalyzed aniline additions to styrenes, see: Kawatsura M, Hartwig JF. J. Am. Chem. Soc. 2000;122:9546.Hu A, Ogasawara M, Sakamoto T, Okada A, Nakajima K, Takahashi T, Lin W. Adv. Synth. Catal. 2006;348:2051.
-
-
-
For mechanistic studies of Pd-catalyzed styrene and diene hydroamination with anilines, see: Nettekoven U, Hartwig JF. J. Am. Chem. Soc. 2002;124:1166.Johns AM, Utsunomiya M, Incarvito CD, Hartwig JF. J. Am. Chem. Soc. 2006;128:1828. For a study with a Ni catalyst and alkyl amines, see: Pawlas J, Nakao Y, Kawatsura M, Hartwig JF. J. Am. Chem. Soc. 2002;124:3669.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources