Pooled Analysis of the Prognostic and Predictive Effects of TP53 Comutation Status Combined With KRAS or EGFR Mutation in Early-Stage Resected Non-Small-Cell Lung Cancer in Four Trials of Adjuvant Chemotherapy
- PMID: 28453411
- PMCID: PMC6075828
- DOI: 10.1200/JCO.2016.71.2893
Pooled Analysis of the Prognostic and Predictive Effects of TP53 Comutation Status Combined With KRAS or EGFR Mutation in Early-Stage Resected Non-Small-Cell Lung Cancer in Four Trials of Adjuvant Chemotherapy
Abstract
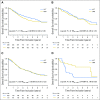
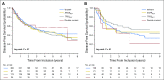
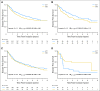
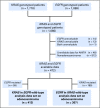
Purpose Our previous work evaluated individual prognostic and predictive roles of TP53, KRAS, and EGFR in non-small-cell lung cancer (NSCLC). In this analysis, we explore the prognostic and predictive roles of TP53/KRAS and TP53/EGFR comutations in randomized trials of adjuvant chemotherapy versus observation. Patients and Methods Mutation analyses (wild-type [WT] and mutant) for TP53, KRAS, and EGFR were determined in blinded fashion in multiple laboratories. Primary and secondary end points of pooled analysis were overall survival and disease-free survival. We evaluated the role of TP53/KRAS comutation in all patients and in the adenocarcinoma subgroup as well as the TP53/EGFR comutation in adenocarcinoma only through a multivariable Cox proportional hazards model stratified by trial. Results Of 3,533 patients with NSCLC, 1,181 (557 deaths) and 404 (170 deaths) were used for TP53/KRAS and TP53/EGFR analyses. For TP53/KRAS mutation status, no prognostic effect was observed ( P = .61), whereas a borderline predictive effect ( P = .04) was observed with a deleterious effect of chemotherapy with TP53/KRAS comutations versus WT/WT (hazard ratio, 2.49 [95% CI, 1.10 to 5.64]; P = .03). TP53/EGFR comutation in adenocarcinoma was neither prognostic ( P = .83), nor significantly predictive ( P = .86). Similar results were observed for both groups for disease-free survival. Conclusion We could identify no prognostic effect of the KRAS or EGFR driver and TP53 tumor suppressor comutation. Our observation of a potential negative predictive effect of TP53/KRAS comutation requires validation.
Figures




References
-
- Harvey JJ. An unidentified virus which causes the rapid production of tumours in mice. Nature. 1964;204:1104–1105. - PubMed
-
- Rodenhuis S, van de Wetering ML, Mooi WJ, et al. Mutational activation of the K-ras oncogene. A possible pathogenetic factor in adenocarcinoma of the lung. N Engl J Med. 1987;317:929–935. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

