Specific blockade of Rictor-mTOR association inhibits mTORC2 activity and is cytotoxic in glioblastoma
- PMID: 28453552
- PMCID: PMC5409528
- DOI: 10.1371/journal.pone.0176599
Specific blockade of Rictor-mTOR association inhibits mTORC2 activity and is cytotoxic in glioblastoma
Erratum in
-
Correction: Specific blockade of Rictor-mTOR association inhibits mTORC2 activity and is cytotoxic in glioblastoma.PLoS One. 2019 Feb 6;14(2):e0212160. doi: 10.1371/journal.pone.0212160. eCollection 2019. PLoS One. 2019. PMID: 30726300 Free PMC article.
Retraction in
-
Retraction: Specific blockade of Rictor-mTOR association inhibits mTORC2 activity and is cytotoxic in glioblastoma.PLoS One. 2023 Sep 8;18(9):e0291490. doi: 10.1371/journal.pone.0291490. eCollection 2023. PLoS One. 2023. PMID: 37682814 Free PMC article. No abstract available.
Abstract
A small molecule which specifically blocks the interaction of Rictor and mTOR was identified utilizing a high-throughput yeast two-hybrid screen and evaluated as a potential inhibitor of mTORC2 activity in glioblastoma multiforme (GBM). In vitro, CID613034 inhibited mTORC2 kinase activity at submicromolar concentrations and in cellular assays specifically inhibited phosphorylation of mTORC2 substrates, including AKT (Ser-473), NDRG1 (Thr-346) and PKCα (Ser-657), while having no appreciable effects on the phosphorylation status of the mTORC1 substrate S6K (Thr-389) or mTORC1-dependent negative feedback loops. CID613034 demonstrated significant inhibitory effects on cell growth, motility and invasiveness in GBM cell lines and sensitivity correlated with relative Rictor or SIN1 expression. Structure-activity relationship analyses afforded an inhibitor, JR-AB2-011, with improved anti-GBM properties and blocked mTORC2 signaling and Rictor association with mTOR at lower effective concentrations. In GBM xenograft studies, JR-AB2-011 demonstrated significant anti-tumor properties. These data support mTORC2 as a viable therapeutic target in GBM and suggest that targeting protein-protein interactions critical for mTORC2 function is an effective strategy to achieve therapeutic responses.
Conflict of interest statement
Figures
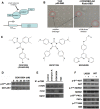
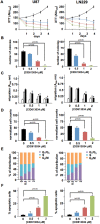
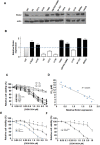
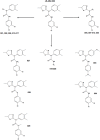
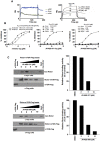
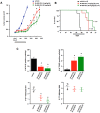
References
-
- Dunn GP, Rinne ML, Wykosky J, Genovese G, Quayle SN, Dunn IF, et al. Emerging insights into the molecular and cellular basis of glioblastoma. Genes Dev. 2012;26(8):756–84. PubMed Central PMCID: PMCPMC3337451. doi: 10.1101/gad.187922.112 - DOI - PMC - PubMed
-
- Cloughesy TF, Cavenee WK, Mischel PS. Glioblastoma: from molecular pathology to targeted treatment. Annual review of pathology. 2014;9:1–25. Epub 2013/08/14. doi: 10.1146/annurev-pathol-011110-130324 - DOI - PubMed
-
- Fan QW, Weiss WA. Targeting the RTK-PI3K-mTOR axis in malignant glioma: overcoming resistance. Curr Top Microbiol Immunol. 2010;347:279–96. PubMed Central PMCID: PMCPMC3012004. doi: 10.1007/82_2010_67 - DOI - PMC - PubMed
-
- Westphal M, Lamszus K. The neurobiology of gliomas: from cell biology to the development of therapeutic approaches. Nat Rev Neurosci. 2011;12(9):495–508. Epub 2011/08/04. doi: 10.1038/nrn3060 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

