Patient-Reported Physical Function Measures in Cancer Clinical Trials
- PMID: 28453627
- PMCID: PMC5858035
- DOI: 10.1093/epirev/mxx008
Patient-Reported Physical Function Measures in Cancer Clinical Trials
Abstract
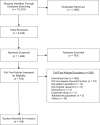
Patient-reported outcomes (PROs) are increasingly used to monitor treatment-related symptoms and physical function decrements in cancer clinical trials. As more patients enter survivorship, it is important to capture PRO physical function throughout trials to help restore pretreatment levels of function. We completed a systematic review of PRO physical function measures used in cancer clinical trials and evaluated their psychometric properties on the basis of guidelines from the US Food and Drug Administration. Five databases were searched through October 2015: PubMed/MEDLINE, EMBASE, CINAHL (Cumulative Index of Nursing and Allied Health Literature), Health and Psychosocial Instruments, and Cochrane. From an initial total of 10,233 articles, we identified 108 trials that captured PRO physical function. Within these trials, approximately 67% used the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire and 25% used the Medical Outcomes Study Short Form 36. Both the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire and Medical Outcomes Study Short Form 36 instruments generically satisfy most Food and Drug Administration requirements, although neither sought direct patient input as part of item development. The newer Patient-Reported Outcomes Measurement Information System physical function short form may be a brief, viable alternative. Clinicians should carefully consider the psychometric properties of these measures when incorporating PRO instrumentation into clinical trial design to provide a more comprehensive understanding of patient function.
Keywords: clinical outcome assessment; health status; neoplasms; patient-reported outcomes.
© The Author 2017. Published by Oxford University Press on behalf of the Johns Hopkins Bloomberg School of Public Health. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Basch E. New frontiers in patient-reported outcomes: adverse event reporting, comparative effectiveness, and quality assessment. Annu Rev Med. 2014;65:307–317. - PubMed
-
- Basch E, Abernethy AP, Mullins CD, et al. . Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30(34):4249–4255. - PubMed
-
- Basch E, Geoghegan C, Coons SJ, et al. . Patient-reported outcomes in cancer drug development and US regulatory review: perspectives from industry, the food and drug administration, and the patient. JAMA Oncol. 2015;1(3):375–379. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous