Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with recurrent carcinoma of the anal canal
- PMID: 28453692
- PMCID: PMC5406758
- DOI: 10.1093/annonc/mdx029
Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with recurrent carcinoma of the anal canal
Abstract
Background: Safety and efficacy of pembrolizumab, a humanized programmed death 1 monoclonal antibody, was assessed in KEYNOTE-028, a multicohort, phase Ib trial for patients with programmed death ligand 1 (PD-L1)-positive advanced solid tumors. We report results for the cohort of patients with advanced anal carcinoma.
Patients and methods: Patients with PD-L1-positive tumors (≥1%) received intravenous pembrolizumab 10 mg/kg once every 2 weeks for up to 2 years or until confirmed progression or unacceptable toxicity. Response was assessed every 8 weeks for the first 6 months and every 12 weeks thereafter per Response Evaluation Criteria In Solid Tumors, version 1.1. Primary endpoints were safety and overall response rate per investigator review. Secondary endpoints included progression-free survival, overall survival, and response duration. Data cutoff date was 1 July 2015.
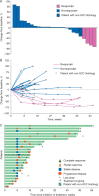
Results: Of the 43 patients with advanced anal carcinoma evaluable for PD-L1 expression, 32 (74%) had PD-L1-positive tumors as assessed with the 22C3 prototype assay, of whom 25 were enrolled between April and September 2014. Sixteen patients (64%) experienced treatment-related adverse events; the most common ones were diarrhea and fatigue in four patients (16%) each and nausea in three patients (12%). There were no treatment-related deaths or discontinuations as of the data cutoff date. Among the 24 patients with squamous cell carcinoma histology, four had confirmed partial response, for an overall response rate of 17% [95% confidence interval (CI), 5%-37%) and 10 (42%) had confirmed stable disease, for a disease control rate of 58%. One additional patient with non-squamous histology had confirmed stable disease.
Conclusion: In this population of patients with PD-L1-positive advanced squamous cell anal carcinoma, pembrolizumab demonstrated a manageable safety profile and encouraging antitumor activity. These data support further study of pembrolizumab for this patient population.
Clinicaltrials.gov: NCT02054806.
Keywords: KEYNOTE-028; PD-1; PD-L1; immunotherapy; pembrolizumab; squamous cell advanced anal carcinoma.
© The Author 2017. Published by Oxford University Press on behalf of the European Society for Medical Oncology.
Figures
Comment in
-
Treatment of squamous cell carcinoma of the anal canal (SCCA): a new era?Ann Oncol. 2017 Oct 1;28(10):2620. doi: 10.1093/annonc/mdx291. Ann Oncol. 2017. PMID: 28945890 No abstract available.
-
Anal cancer and immunotherapy-are we there yet?Transl Gastroenterol Hepatol. 2019 Aug 19;4:57. doi: 10.21037/tgh.2019.08.02. eCollection 2019. Transl Gastroenterol Hepatol. 2019. PMID: 31559338 Free PMC article. No abstract available.
References
-
- Grulich AE, Poynten IM, Machalek DA. et al. The epidemiology of anal cancer. Sex Heal 2012; 9: 504–508. - PubMed
-
- De Vuyst H, Clifford GM, Nascimento MC. et al. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer 2009; 124: 1626–1636. - PubMed
-
- Bartelink H, Roelofsen F, Eschwege F. et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol 1997; 15: 2040–2049. - PubMed
-
- Flam M, John M, Pajak TF. et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol 1996; 14: 2527–2539. - PubMed
-
- Ajani JA, Winter KA, Gunderson LL. et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA 2008; 299: 1914–1921. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

