A phase 2, randomized, double-blind, placebo- controlled study of chemo-immunotherapy combination using motolimod with pegylated liposomal doxorubicin in recurrent or persistent ovarian cancer: a Gynecologic Oncology Group partners study
- PMID: 28453702
- PMCID: PMC5406764
- DOI: 10.1093/annonc/mdx049
A phase 2, randomized, double-blind, placebo- controlled study of chemo-immunotherapy combination using motolimod with pegylated liposomal doxorubicin in recurrent or persistent ovarian cancer: a Gynecologic Oncology Group partners study
Abstract
Background: A phase 2, randomized, placebo-controlled trial was conducted in women with recurrent epithelial ovarian carcinoma to evaluate the efficacy and safety of motolimod-a Toll-like receptor 8 (TLR8) agonist that stimulates robust innate immune responses-combined with pegylated liposomal doxorubicin (PLD), a chemotherapeutic that induces immunogenic cell death.
Patients and methods: Women with ovarian, fallopian tube, or primary peritoneal carcinoma were randomized 1 : 1 to receive PLD in combination with blinded motolimod or placebo. Randomization was stratified by platinum-free interval (≤6 versus >6-12 months) and Gynecologic Oncology Group (GOG) performance status (0 versus 1). Treatment cycles were repeated every 28 days until disease progression.
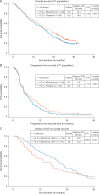
Results: The addition of motolimod to PLD did not significantly improve overall survival (OS; log rank one-sided P = 0.923, HR = 1.22) or progression-free survival (PFS; log rank one-sided P = 0.943, HR = 1.21). The combination was well tolerated, with no synergistic or unexpected serious toxicity. Most patients experienced adverse events of fatigue, anemia, nausea, decreased white blood cells, and constipation. In pre-specified subgroup analyses, motolimod-treated patients who experienced injection site reactions (ISR) had a lower risk of death compared with those who did not experience ISR. Additionally, pre-treatment in vitro responses of immune biomarkers to TLR8 stimulation predicted OS outcomes in patients receiving motolimod on study. Immune score (tumor infiltrating lymphocytes; TIL), TLR8 single-nucleotide polymorphisms, mutational status in BRCA and other DNA repair genes, and autoantibody biomarkers did not correlate with OS or PFS.
Conclusions: The addition of motolimod to PLD did not improve clinical outcomes compared with placebo. However, subset analyses identified statistically significant differences in the OS of motolimod-treated patients on the basis of ISR and in vitro immune responses. Collectively, these data may provide important clues for identifying patients for treatment with immunomodulatory agents in novel combinations and/or delivery approaches.
Trial registration: Clinicaltrials.gov, NCT 01666444.
Trial registration: ClinicalTrials.gov NCT01666444.
Keywords: Toll-like receptor 8; biomarkers; immunotherapy; motolimod; oncology; ovarian cancer.
© The Author 2017. Published by Oxford University Press on behalf of the European Society for Medical Oncology.
Figures


Comment in
-
Immunotherapy and epithelial ovarian cancer: a double-edged sword?Ann Oncol. 2017 May 1;28(5):909-910. doi: 10.1093/annonc/mdx102. Ann Oncol. 2017. PMID: 28379282 No abstract available.
References
-
- American Cancer Society. What are the key statistics about ovarian cancer? [article on internet] 2016. https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html (22 September 2016, date last accessed).
-
- NIH National Cancer Institute. SEER Stat Fact Sheets: Ovarian Cancer [database on internet] 2016. https://seer.cancer.gov/statfacts/html/ovary.html (9 November 2016, date last accessed).
-
- Morgan RJ Jr, Armstrong DK, Alvarez RD. et al. Ovarian cancer, Version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016; 14: 1134–1163. - PubMed
-
- Buda A, Floriani I, Rossi R. et al. Randomised controlled trial comparing single agent paclitaxel vs epidoxorubicin plus paclitaxel in patients with advanced ovarian cancer in early progression after platinum-based chemotherapy: an Italian Collaborative Study from the Mario Negri Institute, Milan, G.O.N.O. (Gruppo Oncologico Nord Ovest) group and I.O.R. (Istituto Oncologico Romagnolo) group. Br J Cancer 2004; 90: 2112–2117. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

