Targeted next-generation sequencing of pediatric neuro-oncology patients improves diagnosis, identifies pathogenic germline mutations, and directs targeted therapy
- PMID: 28453743
- PMCID: PMC5464451
- DOI: 10.1093/neuonc/now254
Targeted next-generation sequencing of pediatric neuro-oncology patients improves diagnosis, identifies pathogenic germline mutations, and directs targeted therapy
Erratum in
-
Corrigenda.Neuro Oncol. 2017 Apr 1;19(4):601. doi: 10.1093/neuonc/nox024. Neuro Oncol. 2017. PMID: 28388712 Free PMC article. No abstract available.
Abstract
Background: Molecular profiling is revolutionizing cancer diagnostics and leading to personalized therapeutic approaches. Herein we describe our clinical experience performing targeted sequencing for 31 pediatric neuro-oncology patients.
Methods: We sequenced 510 cancer-associated genes from tumor and peripheral blood to identify germline and somatic mutations, structural variants, and copy number changes.
Results: Genomic profiling was performed on 31 patients with tumors including 11 high-grade gliomas, 8 medulloblastomas, 6 low-grade gliomas, 1 embryonal tumor with multilayered rosettes, 1 pineoblastoma, 1 uveal ganglioneuroma, 1 choroid plexus carcinoma, 1 chordoma, and 1 high-grade neuroepithelial tumor. In 25 cases (81%), results impacted patient management by: (i) clarifying diagnosis, (ii) identifying pathogenic germline mutations, or (iii) detecting potentially targetable alterations. The pathologic diagnosis was amended after genomic profiling for 6 patients (19%), including a high-grade glioma to pilocytic astrocytoma, medulloblastoma to pineoblastoma, ependymoma to high-grade glioma, and medulloblastoma to CNS high-grade neuroepithelial tumor with BCOR alteration. Multiple patients had pathogenic germline mutations, many of which were previously unsuspected. Potentially targetable alterations were identified in 19 patients (61%). Additionally, novel likely pathogenic alterations were identified in 3 cases: an in-frame RAF1 fusion in a BRAF wild-type pleomorphic xanthoastrocytoma, an inactivating ASXL1 mutation in a histone H3 wild-type diffuse pontine glioma, and an in-frame deletion within exon 2 of MAP2K1 in a low-grade astrocytic neoplasm.
Conclusions: Our experience demonstrates the significant impact of molecular profiling on diagnosis and treatment of pediatric brain tumors and confirms its feasibility for use at the time of diagnosis or recurrence.
Keywords: molecular profiling; next-generation sequencing; pediatric brain tumors; personalized therapy; targeted therapeutics.
© The Author(s) 2016. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com
Figures
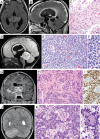
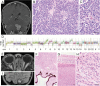
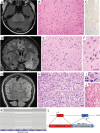
References
-
- Broniscer A, Gajjar A. Supratentorial high-grade astrocytoma and diffuse brainstem glioma: two challenges for the pediatric oncologist. Oncologist. 2004;9(2):197–206. - PubMed
-
- Northcott PA, Pfister SM, Jones DT. Next-generation (epi)genetic drivers of childhood brain tumours and the outlook for targeted therapies. Lancet Oncol. 2015;16(6):e293–e302. - PubMed
-
- Sahm F, Schrimpf D, Jones DT, et al. Next-generation sequencing in routine brain tumor diagnostics enables an integrated diagnosis and identifies actionable targets. Acta Neuropathol. 2016;131(6):903–910. - PubMed
-
- Hoffman LM, Salloum R, Fouladi M. Molecular biology of pediatric brain tumors and impact on novel therapies. Curr Neurol Neurosci Rep. 2015;15(4):10. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

