JS-K promotes apoptosis by inducing ROS production in human prostate cancer cells
- PMID: 28454225
- PMCID: PMC5403315
- DOI: 10.3892/ol.2016.5535
JS-K promotes apoptosis by inducing ROS production in human prostate cancer cells
Abstract
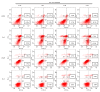
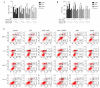
Reactive oxygen species (ROS) are chemical species that alter redox status, and are responsible for inducing carcinogenesis. The purpose of the present study was to assess the effects of the glutathione S transferase-activated nitric oxide donor prodrug, JS-K, on ROS accumulation and on proliferation and apoptosis in human prostate cancer cells. Cell proliferation and apoptosis, ROS accumulation and the activation of the mitochondrial signaling pathway were measured. The results demonstrated that JS-K may inhibit prostate cancer cell growth in a dose- and time-dependent manner, and induce ROS accumulation and apoptosis in a dose-dependent manner. With increasing concentrations of JS-K, expression of pro-apoptotic proteins increased, but Bcl-2 expression decreased. Additionally, the antioxidant N-acetylcysteine reversed JS-K-induced cell apoptosis; conversely, the pro-oxidant glutathione disulfide exacerbated JS-K-induced apoptosis. In conclusion, the data suggest that JS-K induces prostate cancer cell apoptosis by increasing ROS levels.
Keywords: JS-K; apoptosis; nitric oxide; prostate cancer cells; reactive oxygen species.
Figures


References
-
- Shami PJ, Saavedra JE, Wang LY, Bonifant CL, Diwan BA, Singh SV, Gu Y, Fox SD, Buzard GS, Citro ML, et al. JS-K, a glutathione/glutathione S-transferase-activated nitric oxide donor of the diazeniumdiolate class with potent antineoplastic activity. Mol Cancer Ther. 2003;2:409–417. - PubMed
-
- Kiziltepe T, Hideshima T, Ishitsuka K, Ocio EM, Raje N, Catley L, Li CQ, Trudel LJ, Yasui H, Vallet S, et al. JS-K, a GST-activated nitric oxide generator, induces DNA double-strand breaks, activates DNA damage response pathways, and induces apoptosis in vitro and in vivo in human multiple myeloma cells. Blood. 2007;110:709–718. doi: 10.1182/blood-2006-10-052845. - DOI - PMC - PubMed
-
- Maciag AE, Chakrapani H, Saavedra JE, Morris NL, Holland RJ, Kosak KM, Shami PJ, Anderson LM, Keefer LK. The nitric oxide prodrug JS-K is effective against non-small-cell lung cancer cells in vitro and in vivo: Involvement of reactive oxygen species. J Pharmacol Exp Ther. 2011;336:313–320. doi: 10.1124/jpet.110.174904. - DOI - PMC - PubMed
-
- Laschak M, Spindler KD, Schrader AJ, Hessenauer A, Streicher W, Schrader M, Cronauer MV. JS-K, a glutathione/glutathione S-transferase-activated nitric oxide releasing prodrug inhibits androgen receptor and WNT-signaling in prostate cancer cells. BMC Cancer. 2012;12:130. doi: 10.1186/1471-2407-12-130. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
