Cisplatin plus vinorelbine as induction treatment in stage IIIA non-small cell lung cancer
- PMID: 28454304
- PMCID: PMC5403378
- DOI: 10.3892/ol.2017.5620
Cisplatin plus vinorelbine as induction treatment in stage IIIA non-small cell lung cancer
Abstract
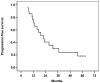
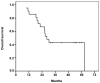
Survival rates in patients with stage IIIA non-small cell lung cancer (NSCLC) remain low despite curative treatment. This is due to tumor recurrence at distant sites. The aim of neoadjuvant chemotherapy (NA-CT) is to eradicate occult micrometastatic disease and improve survival in patients that are not candidates for surgery following induction therapy. A total of 21 patients with ipsilateral mediastinal node involvement (N2) with potentially resectable disease, who had been diagnosed with stage IIIA (T1-3 N1-2 and T4N0) NSCLC and who had received cisplatin and vinorelbine as induction treatment were included in this retrospective study. Patients who responded to the treatment underwent surgery, and those who were unresponsive received radical radiotherapy. Follow-up was conducted between March 2008 and April 2014. The median age of patients was 61 years, and all patients exhibited a good Eastern Cooperative Oncology Group performance status. The majority of patients were histologically diagnosed with adenocarcinoma (48%) or squamous cell carcinoma (38%), which was a poor prognostic factor for overall survival (OS). A total of 7 patients underwent surgery (of which 6 were down-staged), with a 3-year survival rate of 42.8%. The most significant factor associated with response to induction treatment was multistation nodal involvement. The complete resection rate for surgical patients was 85.7%. Unresectable patients had a 3-year survival rate of 25.8%. OS time for the whole cohort was 28.5 months, and the 3- and 5-year OS rates were 28.5% and 4.7%, respectively. CT-induced toxicity did not affect any treatment regime or surgical procedures. In conclusion, the use of cisplatin plus vinorelbine is feasible in a neoadjuvant setting, with good response rates and acceptable toxicity. Multistation N2 involvement is the main prognostic factor for a poor response to induction treatment.
Keywords: cisplatin; induction treatment; neoadjuvant chemotherapy; stage III non-small cell lung cancer; survival; vinorelbine.
Figures
References
-
- American Cancer Society, corp-author. Lung cancer (Non-small cell) Atlanta, GA, USA: 2016.
-
- Lim E, Harris G, Patel A, Adachi I, Edmonds L, Song F. Preoperative versus postoperative chemotherapy in patients with resectable non-small cell lung cancer: Systematic review and indirect comparison meta-analysis of randomized trials. J Thorac Oncol. 2009;4:1380–1388. doi: 10.1097/JTO.0b013e3181b9ecca. - DOI - PubMed
-
- Arriagada R, Dunant A, Pignon JP, Bergman B, Chabowski M, Grunenwald D, Kozlowski M, Le Péchoux C, Pirker R, Pinel MI, et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol. 2010;28(1):35–42. doi: 10.1200/JCO.2009.23.2272. - DOI - PubMed
-
- Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, et al., editors. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 1975–2013. Accessed September 1, 2016.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
