Modeling nasopharyngeal carcinoma in three dimensions
- PMID: 28454359
- PMCID: PMC5403637
- DOI: 10.3892/ol.2017.5697
Modeling nasopharyngeal carcinoma in three dimensions
Abstract
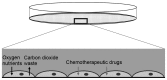
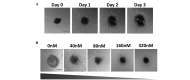
Nasopharyngeal carcinoma (NPC) is a type of cancer endemic in Asia, including Malaysia, Southern China, Hong Kong and Taiwan. Treatment resistance, particularly in recurring cases, remains a challenge. Thus, studies to develop novel therapeutic agents are important. Potential therapeutic compounds may be effectively examined using two-dimensional (2D) cell culture models, three-dimensional (3D) spheroid models or in vivo animal models. The majority of drug assessments for cancers, including for NPC, are currently performed with 2D cell culture models. This model offers economical and high-throughput screening advantages. However, 2D cell culture models cannot recapitulate the architecture and the microenvironment of a tumor. In vivo models may recapitulate certain architectural and microenvironmental conditions of a tumor, however, these are not feasible for the screening of large numbers of compounds. By contrast, 3D spheroid models may be able to recapitulate a physiological microenvironment not observed in 2D cell culture models, in addition to avoiding the impediments of in vivo animal models. Thus, the 3D spheroid model offers a more representative model for the study of NPC growth, invasion and drug response, which may be cost-effective without forgoing quality.
Keywords: 2-dimensional cell culture model; 3-dimensional spheroid model; in vivo models; nasopharyngeal carcinoma; spheroids.
Figures




References
-
- Loh LE, Chee TS, John AB. The anatomy of the Fossa of Rosenmuller-its possible influence on the detection of occult nasopharyngeal carcinoma. Singapore Med J. 1991;32:154–155. - PubMed
-
- Pua KC, Khoo AS, Yap YY, Subramaniam SK, Ong CA, Krishnan G Gopala, Shahid H. Malaysian Nasopharyngeal Carcinoma Study Group: Nasopharyngeal Carcinoma Database. Med J Malaysia. 2008;63:59–62. (Suppl C) - PubMed
-
- Khoo ASB, Pua KC. In: Nasopharyngeal Carcinoma: Keys for Translational Medicine and Biology. Busson P, editor. Landes Bioscience; Austin, TX: 2013. - DOI
-
- Chan AT, Hsu MM, Goh BC, Hui EP, Liu TW, Millward MJ, Hong RL, Whang-Peng J, Ma BB, To KF, et al. Multicenter, phase II study of cetuximab in combination with carboplatin in patients with recurrent or metastatic nasopharyngeal carcinoma. J Clin Oncol. 2005;23:3568–3576. doi: 10.1200/JCO.2005.02.147. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
