A critical assessment of the "sterile womb" and "in utero colonization" hypotheses: implications for research on the pioneer infant microbiome
- PMID: 28454555
- PMCID: PMC5410102
- DOI: 10.1186/s40168-017-0268-4
A critical assessment of the "sterile womb" and "in utero colonization" hypotheses: implications for research on the pioneer infant microbiome
Abstract
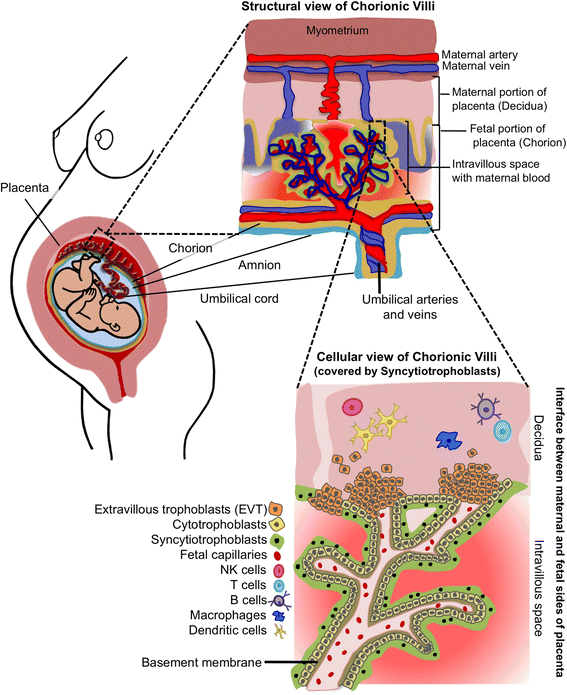
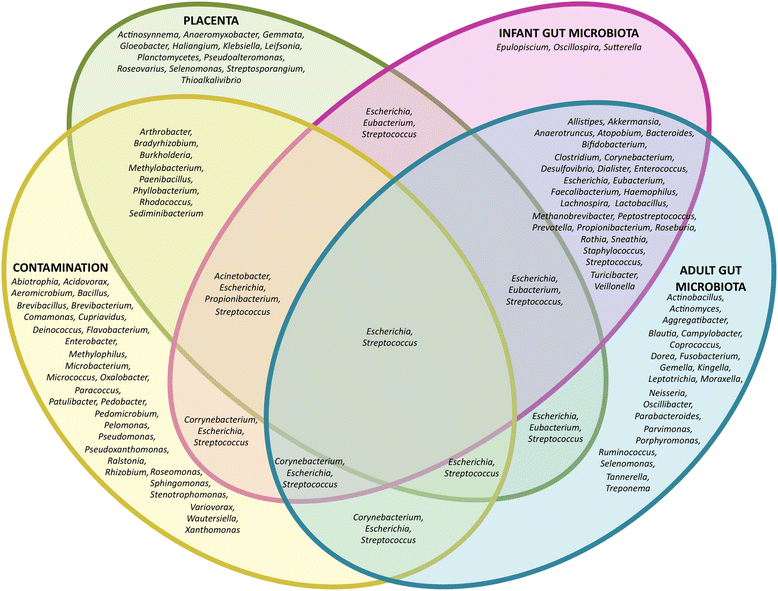
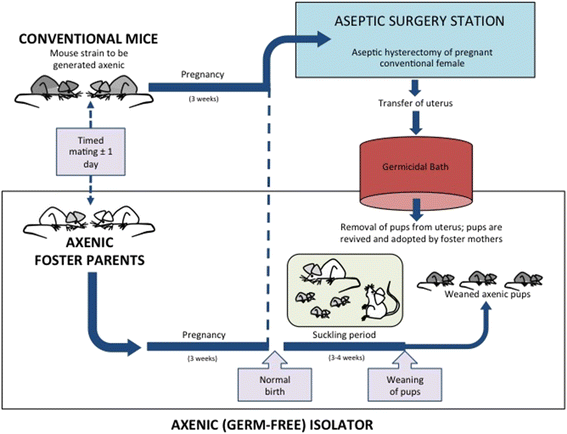
After more than a century of active research, the notion that the human fetal environment is sterile and that the neonate's microbiome is acquired during and after birth was an accepted dogma. However, recent studies using molecular techniques suggest bacterial communities in the placenta, amniotic fluid, and meconium from healthy pregnancies. These findings have led many scientists to challenge the "sterile womb paradigm" and propose that microbiome acquisition instead begins in utero, an idea that would fundamentally change our understanding of gut microbiota acquisition and its role in human development. In this review, we provide a critical assessment of the evidence supporting these two opposing hypotheses, specifically as it relates to (i) anatomical, immunological, and physiological characteristics of the placenta and fetus; (ii) the research methods currently used to study microbial populations in the intrauterine environment; (iii) the fecal microbiome during the first days of life; and (iv) the generation of axenic animals and humans. Based on this analysis, we argue that the evidence in support of the "in utero colonization hypothesis" is extremely weak as it is founded almost entirely on studies that (i) used molecular approaches with an insufficient detection limit to study "low-biomass" microbial populations, (ii) lacked appropriate controls for contamination, and (iii) failed to provide evidence of bacterial viability. Most importantly, the ability to reliably derive axenic animals via cesarean sections strongly supports sterility of the fetal environment in mammals. We conclude that current scientific evidence does not support the existence of microbiomes within the healthy fetal milieu, which has implications for the development of clinical practices that prevent microbiome perturbations after birth and the establishment of future research priorities.
Keywords: Axenic animals; Contamination; In utero colonization; Microbiome; Placenta; Sterile womb.
Figures

References
-
- Arrieta M-C, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, Kuzeljevic B, Gold MJ, Britton HM, Lefebvre DL, Subbarao P, Mandhane P, Becker A, McNagny KM, Sears MR, Kollmann T, Mohn WW, Turvey SE, Brett Finlay B. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7:307ra152. doi: 10.1126/scitranslmed.aab2271. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

