Assisted and Unassisted Protein Insertion into Liposomes
- PMID: 28454841
- PMCID: PMC5607036
- DOI: 10.1016/j.bpj.2017.03.027
Assisted and Unassisted Protein Insertion into Liposomes
Abstract
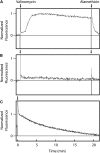
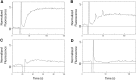
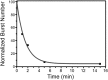
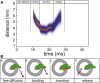
The insertion of newly synthesized membrane proteins is a well-regulated and fascinating process occurring in every living cell. Several translocases and insertases have been found in prokaryotic and eukaryotic cells, the Sec61 complex and the Get complex in the endoplasmic reticulum and the SecYEG complex and YidC in bacteria and archaea. In mitochondria, TOM and TIM complexes transport nuclear-encoded proteins, whereas the Oxa1 is required for the insertion of mitochondria-encoded membrane proteins. Related to the bacterial YidC and the mitochondrial Oxa1 are the Alb3 and Alb4 proteins in chloroplasts. These membrane insertases are comparably simple and can be studied in vitro, after their biochemical purification and reconstitution in artificial lipid bilayers such as liposomes or nanodiscs. Here, we describe the recent progress to study the molecular mechanism of YidC-dependent and unassisted membrane insertion at the single molecule level.
Copyright © 2017 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Castanié-Cornet M.P., Bruel N., Genevaux P. Chaperone networking facilitates protein targeting to the bacterial cytoplasmic membrane. Biochim. Biophys. Acta. 2014;1843:1442–1456. - PubMed
-
- Dudek J., Pfeffer S., Zimmermann R. Protein transport into the human endoplasmic reticulum. J. Mol. Biol. 2015;427(6 pt. A):1159–1175. - PubMed
-
- Park E., Rapoport T.A. Mechanisms of Sec61/SecY-mediated protein translocation across membranes. Annu. Rev. Biophys. 2012;41:21–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

