GOT1-mediated anaplerotic glutamine metabolism regulates chronic acidosis stress in pancreatic cancer cells
- PMID: 28455244
- PMCID: PMC5488721
- DOI: 10.1016/j.canlet.2017.04.029
GOT1-mediated anaplerotic glutamine metabolism regulates chronic acidosis stress in pancreatic cancer cells
Abstract
The increased rate of glycolysis and reduced oxidative metabolism are the principal biochemical phenotypes observed in pancreatic ductal adenocarcinoma (PDAC) that lead to the development of an acidic tumor microenvironment. The pH of most epithelial cell-derived tumors is reported to be lower than that of plasma. However, little is known regarding the physiology and metabolism of cancer cells enduring chronic acidosis. Here, we cultured PDAC cells in chronic acidosis (pH 6.9-7.0) and observed that cells cultured in low pH had reduced clonogenic capacity. However, our physiological and metabolomics analysis showed that cells in low pH deviate from glycolytic metabolism and rely more on oxidative metabolism. The increased expression of the transaminase enzyme GOT1 fuels oxidative metabolism of cells cultured in low pH by enhancing the non-canonical glutamine metabolic pathway. Survival in low pH is reduced upon depletion of GOT1 due to increased intracellular ROS levels. Thus, GOT1 plays an important role in energy metabolism and ROS balance in chronic acidosis stress. Our studies suggest that targeting anaplerotic glutamine metabolism may serve as an important therapeutic target in PDAC.
Keywords: Acidic microenvironment; Anaplerotic glutamine metabolism; Cancer metabolism; Low pH; Pancreatic cancer.
Copyright © 2017 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures
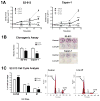

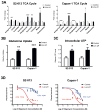

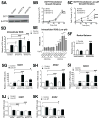

References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
-
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nature reviews Cancer. 2011;11:85–95. - PubMed
-
- Newsholme EA, Crabtree B, Ardawi MS. The role of high rates of glycolysis and glutamine utilization in rapidly dividing cells. Bioscience reports. 1985;5:393–400. - PubMed
-
- Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

