Lamins and nesprin-1 mediate inside-out mechanical coupling in muscle cell precursors through FHOD1
- PMID: 28455503
- PMCID: PMC5430732
- DOI: 10.1038/s41598-017-01324-z
Lamins and nesprin-1 mediate inside-out mechanical coupling in muscle cell precursors through FHOD1
Abstract
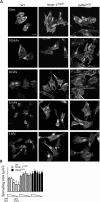
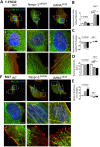
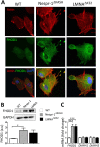
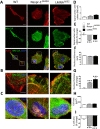
LINC complexes are crucial for the response of muscle cell precursors to the rigidity of their environment, but the mechanisms explaining this behaviour are not known. Here we show that pathogenic mutations in LMNA or SYNE-1 responsible for severe muscle dystrophies reduced the ability of human muscle cell precursors to adapt to substrates of different stiffness. Plated on muscle-like stiffness matrix, mutant cells exhibited contractile stress fibre accumulation, increased focal adhesions, and higher traction force than controls. Inhibition of Rho-associated kinase (ROCK) prevented cytoskeletal defects, while inhibiting myosin light chain kinase or phosphorylation of focal adhesion kinase was ineffective. Depletion or inactivation of a ROCK-dependent regulator of actin remodelling, the formin FHOD1, largely rescued morphology in mutant cells. The functional integrity of lamin and nesprin-1 is thus required to modulate the FHOD1 activity and the inside-out mechanical coupling that tunes the cell internal stiffness to match that of its soft, physiological-like environment.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

