Relationship between carotid plaque surface morphology and perfusion: a 3D DCE-MRI study
- PMID: 28455630
- PMCID: PMC5813060
- DOI: 10.1007/s10334-017-0621-4
Relationship between carotid plaque surface morphology and perfusion: a 3D DCE-MRI study
Abstract
Objective: This study aims to explore the relationship between plaque surface morphology and neovascularization using a high temporal and spatial resolution 4D contrast-enhanced MRI/MRA sequence.
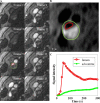
Materials and methods: Twenty one patients with either recent symptoms or a carotid artery stenosis ≥40% were recruited in this study. Plaque surface morphology and luminal stenosis were determined from the arterial phase MRA images. Carotid neovascularization was evaluated by a previously validated pharmacokinetic (PK) modeling approach. K trans (transfer constant) and v p (partial plasma volume) were calculated in both the adventitia and plaque.
Results: Image acquisition and analysis was successfully performed in 28 arteries. Mean luminal stenosis was 44% (range 11-82%). Both adventitial and plaque K trans in ulcerated/irregular plaques were significantly higher than smooth plaques (0.079 ± 0.018 vs. 0.064 ± 0.011 min-1, p = 0.02; 0.065 ± 0.013 vs. 0.055 ± 0.010 min-1, p = 0.03, respectively). Positive correlations between adventitial K trans and v p against stenosis were observed (r = 0.44, p = 0.02; r = 0.55, p = 0.01, respectively).
Conclusion: This study demonstrates the feasibility of using a single sequence to acquire both high resolution 4D CE-MRA and DCE-MRI to evaluate both plaque surface morphology and function. The results demonstrate significant relationships between lumen surface morphology and neovascularization.
Keywords: Carotid atherosclerotic plaque; Contrast enhanced MR angiography (CE-MRA); Dynamic contrast enhanced MRI (DCE-MRI); Neovascularization; Plaque ulceration.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures




References
-
- Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, Creager MA, Culebras A, Eckel RH, Hart RG. Guidelines for the primary prevention of stroke a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(2):517–584. doi: 10.1161/STR.0b013e3181fcb238. - DOI - PubMed
-
- Moore WS, Barnett H, Beebe HG, Bernstein EF, Brener BJ, Brott T, Caplan LR, Day A, Goldstone J, Hobson RW. Guidelines for carotid endarterectomy a multidisciplinary consensus statement from the Ad Hoc Committee, American Heart Association. Stroke. 1995;26(1):188–201. doi: 10.1161/01.STR.26.1.188. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

